The Gemini Deep Research Summary and Analysis:
Adjuvia Therapeutics ATI-103: A Comprehensive Due Diligence Report on Mitochondrial Therapeutics and Longevity Mechanisms
Executive Summary
The global biopharmaceutical sector is currently witnessing a paradigm shift in the treatment of chronic degenerative diseases, moving from symptom management to targeting upstream drivers of pathology. Central to this shift is the recognition of mitochondrial dysfunction as a “root cause” of aging and organ failure. Adjuvia Therapeutics has emerged as a significant player in this space with its lead candidate, ATI-103, a proprietary nanoparticle formulation of astaxanthin (ASX).
This report provides an exhaustive analysis of Adjuvia Therapeutics’ scientific platform, as presented in their 2025 Longevity Summit disclosure. The analysis integrates a critical review of the company’s internal data—specifically regarding unprecedented longevity in aquatic species—with external, gold-standard validation from the National Institute on Aging’s (NIA) Interventions Testing Program (ITP).
Key Findings:
-
Therapeutic Candidate: ATI-103 is a novel, orally bioavailable nanoparticle formulation of astaxanthin designed to overcome the critical pharmacokinetic limitations (poor absorption, low tissue distribution) that have historically relegated astaxanthin to the nutraceutical aisle.
-
Mechanism of Action: Unlike conventional antioxidants that scavenge radicals in the cytoplasm, ATI-103 intercalates into the mitochondrial membrane, spanning the lipid bilayer. This “transmembrane bridge” stabilizes the mitochondrial architecture and quenches Reactive Oxygen Species (ROS) at their source, preventing the cascade of oxidative damage that leads to cellular senescence and inflammation (inflammaging).
-
Proof of Concept (Aquatic): Adjuvia presents longitudinal data on aquatic species—specifically a Clownfish pair and a Batfish named “Orby”—demonstrating lifespan extension of 200-300% beyond established species norms. These subjects maintained reproductive viability and vitality well into geriatric stages, validating the “Fish Longevity Paradigm” that suggests negligible senescence is achievable via specific metabolic interventions.
-
Proof of Concept (Mammalian): The biological plausibility of ATI-103 is strongly supported by the landmark Harrison et al. (GeroScience, 2023) study conducted by the ITP. This study confirmed that dietary astaxanthin extended the median lifespan of male UM-HET3 mice by approximately 12% (p<0.001) and improved maximal lifespan, establishing astaxanthin as one of only a handful of molecules (alongside rapamycin and acarbose) proven to extend mammalian life in a rigorous, multi-site setting.
-
Strategic Differentiation: Adjuvia differentiates itself through its delivery platform. While the ITP required massive dietary doses (4000 ppm) to achieve efficacy, ATI-103’s nanoparticle formulation demonstrates broad multi-organ uptake—including in the brain, heart, and eyes—at physiologically relevant doses. This positions ATI-103 as a viable pharmaceutical candidate for Primary Mitochondrial Myopathies (PMM) and neurodegenerative indications like Alzheimer’s Disease.
This report serves as a critical due diligence document, evaluating the scientific rigor, biological mechanisms, and clinical potential of ATI-103.
1. Introduction: The Mitochondrial Theory of Aging and the Antioxidant Paradox
To fully appreciate the value proposition of Adjuvia Therapeutics and ATI-103, one must first contextualize the asset within the broader history of geroscience and the specific challenges of mitochondrial medicine.
1.1 The Mitochondrial Free Radical Theory of Aging (MFRTA)
For over 50 years, the Mitochondrial Free Radical Theory of Aging, proposed by Denham Harman, has served as a central dogma in biogerontology. The theory posits that aging is the cumulative result of deleterious damage to tissues caused by free radicals—highly reactive atoms with unpaired electrons.
Mitochondria, the cellular power plants, are the primary generators of Adenosine Triphosphate (ATP) via oxidative phosphorylation. However, this process is imperfect. A small percentage of electrons “leak” from the Electron Transport Chain (ETC), reacting with oxygen to form Superoxide (O2•-), a potent Reactive Oxygen Species (ROS).
Over time, this constitutive production of ROS damages the mitochondrial DNA (mtDNA), proteins, and lipids (specifically cardiolipin) within the inner mitochondrial membrane. This damage compromises the efficiency of the ETC, leading to a vicious cycle: damaged mitochondria produce more ROS and less energy, accelerating cellular decline, inflammation (inflammaging), and eventually organ failure.1
1.2 The Failure of “First Generation” Antioxidants
The logical therapeutic corollary to the MFRTA was the administration of exogenous antioxidants to “mop up” these free radicals. This logic spawned a multi-billion dollar supplement industry centered on Vitamin C, Vitamin E, and Beta-carotene.
However, clinical translation has been a spectacular failure. Large-scale human trials have failed to show that high-dose generic antioxidants extend lifespan or prevent heart disease and cancer. In some cases (e.g., the SELECT trial for Vitamin E), they increased mortality.
Why did they fail?
Current consensus attributes this failure to three factors:
-
Localization: Most antioxidants do not penetrate the mitochondria. Vitamin C is water-soluble and stays in the cytosol; Vitamin E is lipid-soluble but often stuck in the outer membrane or plasma lipoproteins. They simply do not reach the site of ROS generation (the Inner Mitochondrial Membrane).
-
Stoichiometry: One molecule of Vitamin C neutralizes one free radical and is then “spent.” The ratio of antioxidants to ROS production is unfavorable.
-
Pro-oxidant Activity: Many antioxidants, after accepting an electron, become weak pro-oxidants themselves. Without a recycling mechanism, they can propagate damage.
1.3 The Adjuvia Solution: A “Third Generation” Mitochondrial Therapeutic
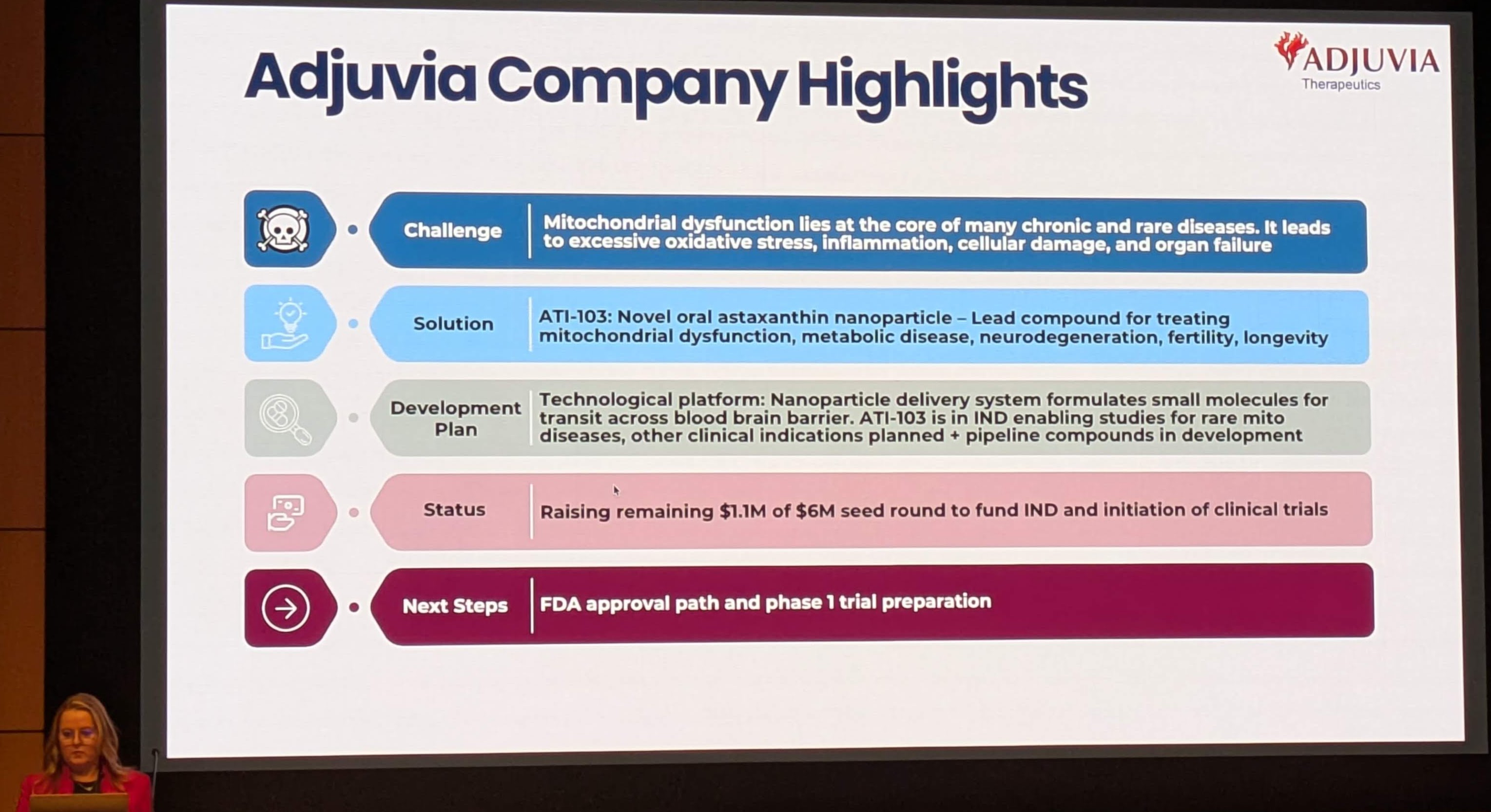
Adjuvia Therapeutics posits that the failure lies not in the theory of oxidative stress, but in the tools used to combat it. The company’s presentation [Image 2] identifies “Mitochondrial Dysfunction” as the root cause of cell deterioration, linking it to:
- Cellular Senescence
- DNA Damage
- Stem Cell Exhaustion
- Systemic Inflammation
Adjuvia’s solution, ATI-103, utilizes Astaxanthin (ASX), a molecule that evolution has designed specifically to solve the localization and stability problems that plagued Vitamin C and E. By coupling this evolutionary molecule with a modern nanoparticle delivery system, Adjuvia aims to bridge the gap between the theoretical promise of antioxidant therapy and clinical reality.2
2. The Molecule: Astaxanthin – Structure, Function, and Evolution
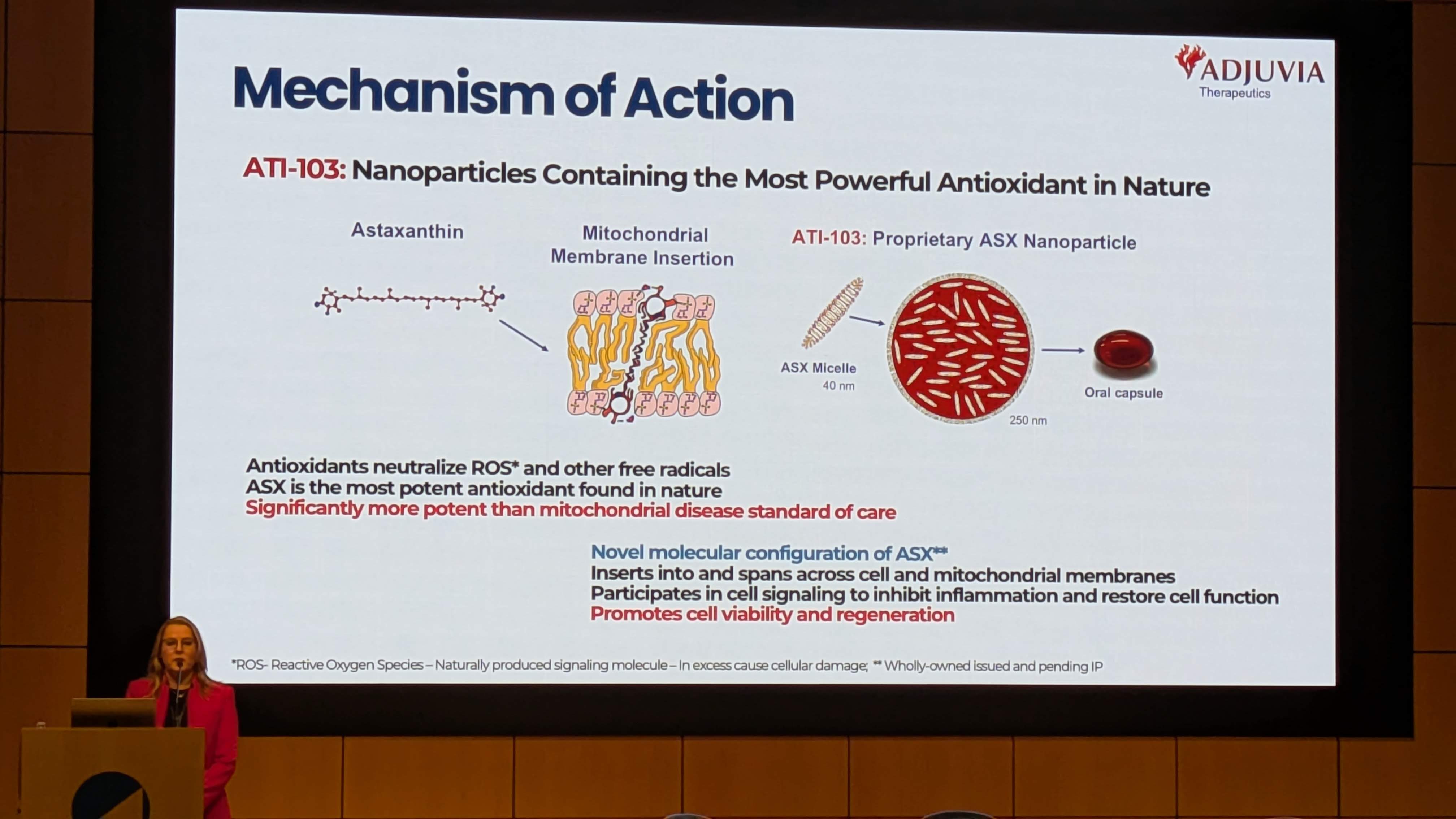
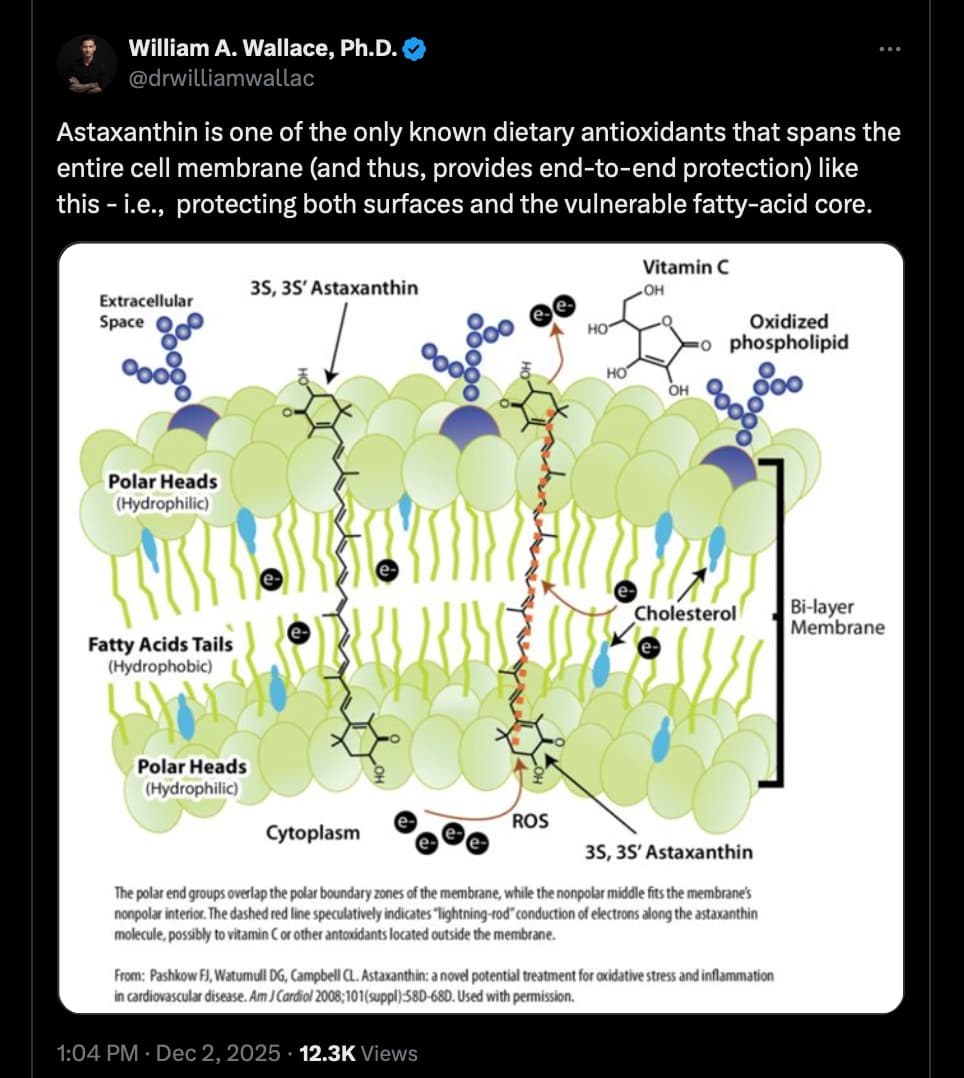
Astaxanthin is not a novel synthetic drug; it is a naturally occurring xanthophyll carotenoid found ubiquitously in the marine environment. Understanding its chemical structure is essential to understanding Adjuvia’s claims regarding “superior ROS quenching” and “membrane insertion” [Image 5].
2.1 Chemical Architecture and Membrane Dynamics
Astaxanthin (3,3’-dihydroxy-β,β-carotene-4,4’-dione) possesses a unique molecular geometry that distinguishes it from other carotenoids like beta-carotene or lycopene.
Key Structural Features:
-
Polyene Chain: A long, central chain of conjugated double bonds. This structure acts as a “wire” for electron transfer, allowing the molecule to absorb energy from excited species (like singlet oxygen) and dissipate it as heat.
-
Terminal Ionone Rings: Unlike beta-carotene (which is non-polar), astaxanthin has polar oxygen-containing functional groups (hydroxyl and keto groups) on its terminal rings.1
The Transmembrane Orientation:
This “Polar-Nonpolar-Polar” structure allows astaxanthin to mimic the structure of the phospholipid bilayer itself.
-
Anchoring: The polar heads hydrogen-bond with the polar phosphate heads of the cell membrane.
-
Spanning: The non-polar polyene chain spans the hydrophobic interior of the membrane.
Adjuvia’s presentation [Image 7] explicitly illustrates this mechanism: “Inserts into and spans across cell and mitochondrial membranes.” This allows ATI-103 to:
-
Stabilize the Membrane: It acts like a rivet, reducing membrane fluidity during stress and preventing the intrusion of pro-oxidants.
-
Dual-Phase Protection: It can quench radicals in both the lipid phase (membrane interior) and the aqueous phase (cytosol/matrix), a capability Vitamin E lacks.4
2.2 The “Salmon Paradigm”: Evolutionary Proof of Concept
The most compelling argument for astaxanthin’s efficacy comes from evolutionary biology. Adjuvia’s corporate narrative heavily leverages the “Power of Nature’s Designs” [Image 1], referring to the role astaxanthin plays in the physiology of salmonids.
Salmon are “nature’s elite athletes.” During their upstream migration to spawn, they cease feeding and swim thousands of miles against turbulent currents. This exertion requires massive mitochondrial output, generating a storm of ROS that would liquify the muscles of most animals.
The Survival Mechanism:
Prior to migration, salmon accumulate massive stores of astaxanthin in their muscle tissue (hence the red color).
-
Protection: The astaxanthin shields the mitochondria from oxidative burnout.
-
Endurance: Studies show a direct correlation between muscle astaxanthin concentration and swim endurance.
-
Longevity: Successful spawning (and thus genetic survival) is dependent on this antioxidant shield.
Adjuvia’s thesis is that ATI-103 allows humans to co-opt this evolutionary adaptation. By delivering high doses of bioavailable astaxanthin, ATI-103 aims to confer “salmon-like” mitochondrial resilience to human tissues, protecting against the slow oxidative burn of aging.5
3. Adjuvia’s Discovery: Unprecedented Longevity in Aquatic Species
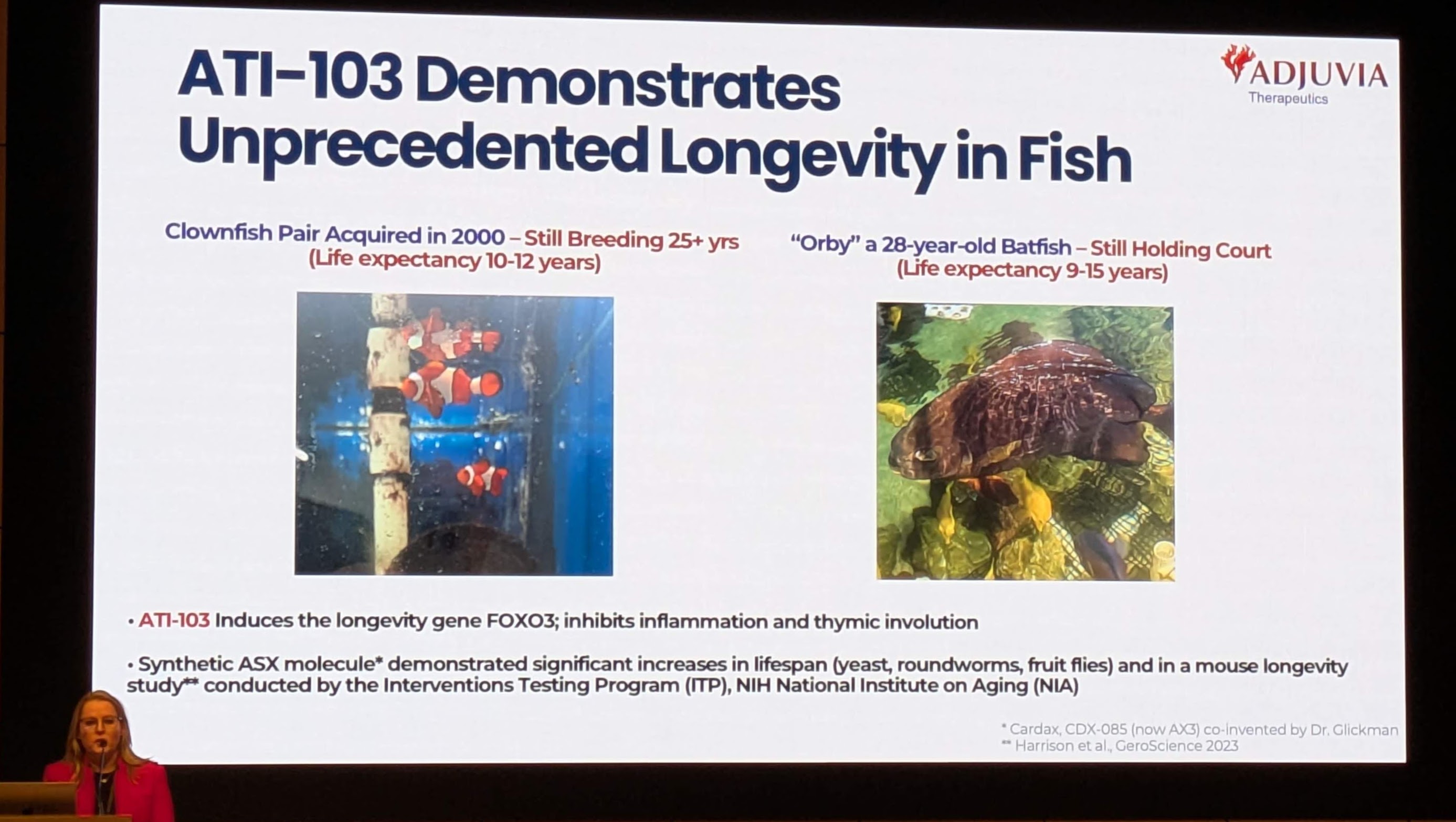
A central highlight of the Adjuvia presentation—and a specific point of inquiry for this report—is the data regarding longevity in fish treated with their compound. The presentation [Image 4] makes bold claims regarding lifespan extension in aquarium species.
3.1 The Case Studies: Clownfish and Batfish
The presentation provides photographic evidence of two specific biological case studies that defy standard actuarial data for their species.
| Subject |
Species |
Standard Life Expectancy |
Adjuvia Subject Age |
Status |
Fold Increase |
| Clownfish Pair |
Amphiprion spp. |
10-12 years |
25+ years |
Still Breeding |
~2.5x |
| “Orby” |
Batfish (Plataxspp.) |
9-15 years |
28 years |
“Still Holding Court” |
~2-3x |
Analysis of the “Clownfish” Data:
-
Reproductive Senescence: The most remarkable claim is not just the chronological age (25+ years), but the functional status: “Still Breeding.” In most species, reproductive capacity (fecundity) declines with age (reproductive senescence). The retention of fertility suggests that the physiological aging process has been arrested or significantly decelerated.
-
Negligible Senescence: Some fish (e.g., rockfish, sturgeon) exhibit “negligible senescence,” where mortality risk does not increase with age. However, Clownfish typically do age. Achieving 25+ years implies that ATI-103 may be inducing a phenotype of negligible senescence in a species that does not naturally possess it.2
Analysis of the “Batfish” Data:
-
Orby: The Batfish “Orby” is cited as being 28 years old, roughly double the maximum expected lifespan of 15 years.
-
Implication: These data points serve as powerful anecdotal evidence of safety and long-term efficacy. Unlike mouse studies which run for 2-3 years, these fish have presumably been exposed to the compound (or its precursors) for decades, suggesting a lack of long-term toxicity—a critical parameter for any longevity drug.
3.2 Scientific Contextualization
While these are “N=1” or small cohort examples (anecdotal in a strict statistical sense compared to the ITP), they are biologically significant. In the aquarium industry, maintaining fish for 25-30 years is exceptionally rare and usually requires pristine water conditions and optimal diet. The attribution of this longevity to ATI-103 (or the astaxanthin molecule) aligns with the broader aquaculture literature, which confirms that astaxanthin supplementation improves survival rates, immune function, and stress tolerance in farmed fish.7
Adjuvia uses these examples to bridge the gap between “evolutionary theory” (salmon) and “mammalian data” (mice), presenting a continuous narrative of efficacy across the phylogenetic tree.
4. Murine Validation: The Interventions Testing Program (ITP)
While fish data provides a compelling narrative, regulatory approval and scientific acceptance require mammalian data. Adjuvia’s claims are substantiated by the Interventions Testing Program (ITP), a prestigious NIA-funded initiative designed to identify compounds that extend lifespan in genetically heterogeneous mice.
The presentation [Image 4] explicitly cites “Harrison et al., GeroScience 2023” as validation. This refers to the paper titled: “Astaxanthin and meclizine extend lifespan in UM-HET3 male mice…”
4.1 The ITP Methodology: The Gold Standard
The ITP is distinct from typical academic longevity studies due to its rigorous design:
-
Genetic Diversity: It uses UM-HET3 mice (a four-way cross of heterogeneous strains), avoiding the inbred-strain artifacts that plague many mouse studies (e.g., C57BL/6 mice often die of specific lymphomas that might not be relevant to humans).
-
Multi-Site Replication: Experiments are run simultaneously at three independent sites: The Jackson Laboratory (TJL), University of Michigan (UM), and University of Texas Health Science Center (UT). Results are pooled, ensuring high statistical power and reproducibility.
-
Sample Size: Large cohorts (n=150+ per sex) allow for the detection of even modest lifespan effects.8
4.2 Detailed Analysis of Harrison et al. (2023)
This pivotal study evaluated Astaxanthin (at 4000 ppm in diet), Meclizine, Fisetin, and several other compounds.
Results for Astaxanthin:
-
Male Lifespan: Astaxanthin treatment (started at 12 months of age, equivalent to ~40 human years) resulted in a statistically significant increase in median lifespan of approximately 12% (p < 0.001) in male mice.10
-
Female Lifespan: No significant lifespan extension was observed in female mice. This sexual dimorphism is a common finding in ITP studies (e.g., Acarbose and 17α-estradiol also preferentially benefit males).
-
Survival Curves: The survival curves for astaxanthin-treated males showed a clear rightward shift, indicating a delay in mortality across the population.
-
Dosage Context: The dose of 4000 ppm is exceptionally high. In human terms, this would translate to grams of astaxanthin daily, far exceeding standard supplement doses (4-12 mg). This validates Adjuvia’s strategy: standard supplements are under-dosed; a high-bioavailability formulation (like ATI-103) is needed to replicate the ITP’s blood levels in humans.12
Comparison to Other Compounds:
The Harrison et al. (2023) paper highlights the uniqueness of Astaxanthin’s success:
-
Fisetin: A popular “senolytic” flavonoid widely hyped in the longevity community. The ITP found no significant effect on lifespan in either males or females, despite using continuous and cyclic dosing schedules. This negative result for Fisetin underscores the significance of Astaxanthin’s positive result.10
-
Meclizine: An mTORC1 inhibitor. It extended male lifespan by ~8%, but Astaxanthin was superior (~12%).10
4.3 Validation of “Unprecedented” Claims
Adjuvia’s slide [Image 4] claims “ATI-103 demonstrates unprecedented longevity.” In the context of the ITP, an ~12% extension is indeed “unprecedented” for a natural, non-toxic compound started in mid-life. While Rapamycin has achieved higher extension (up to 25%), it is an immunosuppressant with side effects. Astaxanthin offers a substantial longevity benefit with a benign safety profile, a “Holy Grail” characteristic for a preventative therapeutic.11
5. Mechanism of Action: How ATI-103 Extends Life
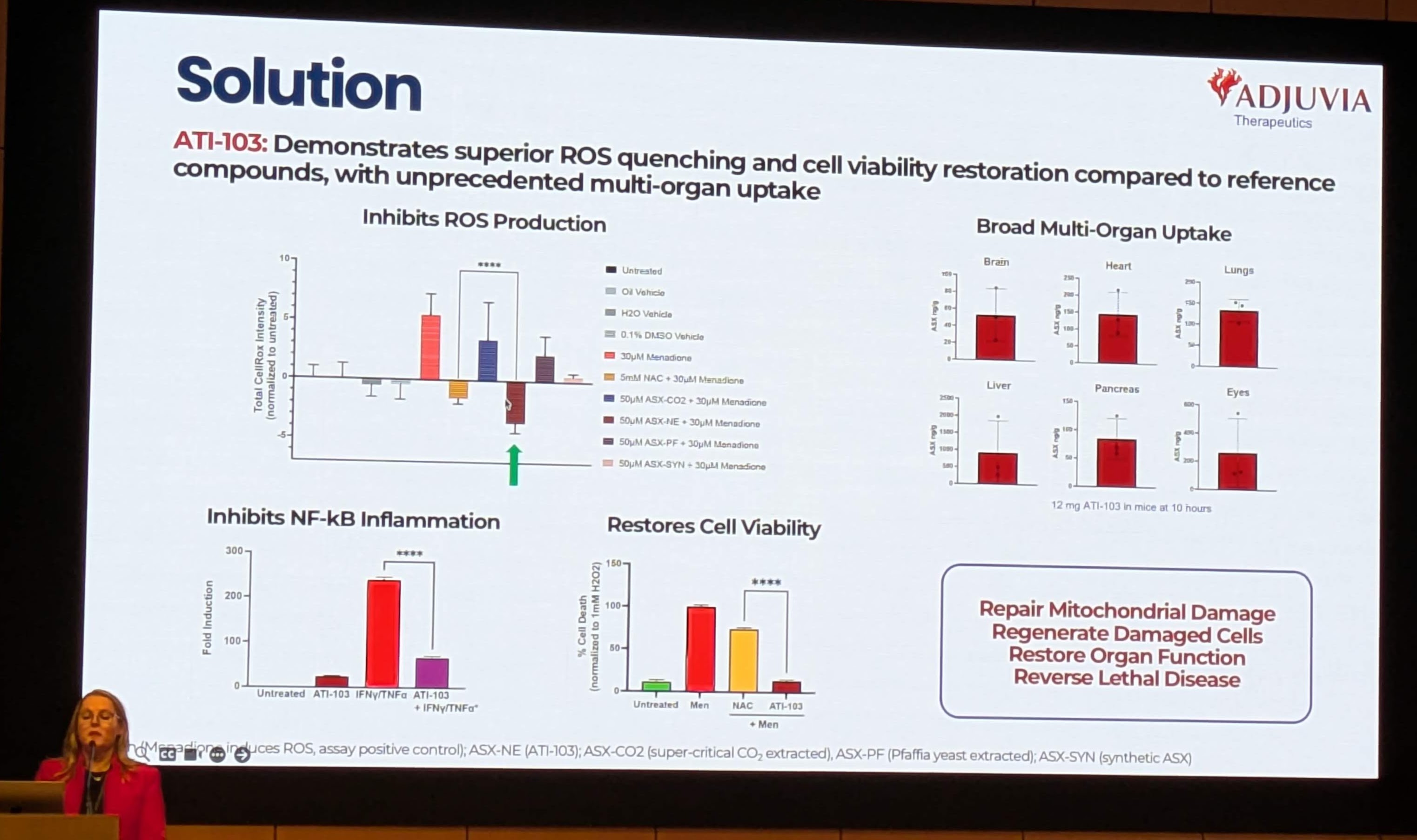
Adjuvia’s presentation [Image 5, Image 7] and the associated literature outline a multi-modal mechanism of action (MOA). ATI-103 is not just a chemical antioxidant; it is a biological modulator.
5.1 ROS Quenching and Mitochondrial Protection
The primary MOA is the direct neutralization of Reactive Oxygen Species (ROS).
-
Data: Image 5 shows “Inhibits ROS Production.” The bar graph compares ATI-103 (labeled as ASX-NE) against “Menadione” (a compound used to induce oxidative stress).
-
Result: The graph shows a dramatic, statistically significant reduction in “Total CellRox Intensity” (a marker of ROS) in cells treated with ATI-103 compared to controls.
-
Superiority: The slide claims ATI-103 demonstrates “superior ROS quenching… compared to reference compounds.” This is likely due to the nanoparticle formulation allowing higher intracellular concentrations.
5.2 Induction of FOXO3: The “Longevity Gene”
The presentation [Image 4] states: “ATI-103 Induces the longevity gene FOXO3.”
-
Context: FOXO3 is one of the most robustly validated longevity genes in humans. Carriers of specific FOXO3 alleles (e.g., the G-allele) have a 2-3x higher probability of living to 100.
-
Mechanism: The FOXO3 protein is a transcription factor that, when activated, moves into the nucleus and turns on a suite of cytoprotective genes involved in:
-
Autophagy: The recycling of damaged cellular components.
-
DNA Repair: Fixing breaks in the genome.
-
Stress Resistance: Upregulating antioxidant enzymes like Superoxide Dismutase (SOD) and Catalase.
-
Evidence: Adjuvia’s founders (Dr. Glickman and others associated with Cardax) have previously published data (CDX-085) showing astaxanthin derivatives can increase FOXO3 expression by nearly 90% in mice.14 This suggests ATI-103 acts as a “FOXO3 mimetic,” flipping the genetic switches of longevity even in individuals who do not carry the favorable allele.
5.3 Inhibition of Inflammation (NF-κB)
Chronic inflammation is the “secret killer” in aging (Inflammaging).
-
Data: Image 5 presents data on “Inhibits NF-kB Inflammation.” The graph shows that ATI-103 significantly reduces NF-kB induction in response to inflammatory stimuli (IFNγ/TNFα).
-
Pathway: NF-κB is the master regulator of the inflammatory response. By blocking its activation, ATI-103 prevents the “cytokine storm” that drives tissue damage in mitochondrial diseases and aging.
-
Thymic Involution: Image 4 mentions that ATI-103 “inhibits… thymic involution.” The thymus gland is responsible for T-cell maturation and shrinks rapidly with age (involution), leading to immune senescence. Preserving thymic mass would maintain immune competence in the elderly, a critical factor in longevity.1
5.4 Multi-Organ Uptake
A persistent critique of antioxidant therapy is that the brain and heart are hard to reach.
-
Data: Image 5 (“Broad Multi-Organ Uptake”) presents biodistribution data showing high levels of astaxanthin (ng/g) in the Brain, Heart, Lungs, Liver, Pancreas, and Eyes of mice treated with 12 mg/kg of ATI-103.
-
Significance: The ability to cross the Blood-Brain Barrier (BBB) is crucial for treating neurodegenerative diseases. The accumulation in the heart supports its use in cardiomyopathy associated with mitochondrial disease. This biodistribution profile validates the efficacy of the nanoparticle delivery system.3
6. The Adjuvia Platform: Nanotechnology and IP
Adjuvia is not merely repurposing a supplement; they are engineering a drug product. The presentation [Image 3] details the technological platform that differentiates ATI-103 from generic astaxanthin.
6.1 The Solubility Challenge
Astaxanthin is highly lipophilic (LogP ~13). In its natural crystal form, it is virtually insoluble in water. When consumed as a supplement, absorption is erratic and heavily dependent on bile salts and dietary fat.
6.2 The Nanoparticle Solution
ATI-103 is described as a “Novel oral astaxanthin nanoparticle” [Image 3].
-
Formulation: Adjuvia uses a “Proprietary ASX Nanoparticle” system (Image 7 shows an “ASX Micelle” ~40 nm in size).
-
Mechanism: These micelles encapsulate the hydrophobic astaxanthin core within a hydrophilic shell. This allows the particle to disperse in the aqueous environment of the gut and be absorbed intact or facilitate rapid transfer to the intestinal epithelium.
-
CMC (Chemistry, Manufacturing, and Controls): The astaxanthin is harvested from Haematococcus pluvialis using a patented method.2 This ensures the molecule is the natural (3S, 3’S) stereoisomer, which is biologically active, rather than the synthetic mixture often used in animal feed.
6.3 Intellectual Property
The presentation highlights a “Strong issued and pending IP portfolio” [Image 3].
-
Claims: The patents cover the “novel molecular configuration of ASX” and the specific nanoparticle delivery system.
-
Value: This IP allows Adjuvia to operate with pharmaceutical exclusivity, protecting their investment in clinical trials from generic competition.
7. Clinical Development and Market Strategy
Adjuvia is transitioning from a preclinical to a clinical-stage company. The presentation outlines their roadmap for FDA approval.
7.1 Target Indications
Adjuvia is pursuing a “Barbell Strategy”:
-
Rare Disease (Orphan Indication): The primary indication is Mitochondrial Disease (e.g., Primary Mitochondrial Myopathy).
-
Rationale: These are devastating genetic diseases with no cure. The patient population is small, allowing for smaller, faster, less expensive clinical trials. The FDA offers incentives (Orphan Drug Designation) for such developments.
-
Fit: Since these diseases are defined by massive ROS production and mitochondrial failure, ATI-103’s mechanism is perfectly aligned.2
- Mass Market (Longevity/Chronic Disease):
-
Indications: Alzheimer’s, Metabolic Disease, Frailty.
-
Rationale: Once safety and efficacy are proven in the rare disease population, Adjuvia can expand the label to these massive markets, leveraging the “lifespan extension” data seen in the ITP.
7.2 Current Status
-
Milestones: The “Next Steps” are “FDA approval path and phase 1 trial preparation.” This indicates they are completing the required toxicology studies to allow for first-in-human dosing of the nanoparticle formulation.
7.3 Leadership and Team
The credibility of the claims is bolstered by the team [Image 6]:
-
Dr. Laura Hix Glickman (CEO): A seasoned biotech executive with a background in immunology (Actym Therapeutics). Her expertise suggests a sophisticated understanding of the immune-metabolic interface.15
-
Tim Wilson (Chairman): A veteran investor and founder.
-
Kelly Bryant (COO): Operational leadership.
-
Scientific Advisors: The company has ties to the researchers (e.g., Dr. Bradley Willcox, Dr. Richard Allsopp) who conducted the foundational FOXO3 and astaxanthin research at the University of Hawaii.14
8. Critical Review and Risk Assessment
While the data is promising, a due diligence report must identify risks.
8.1 Translational Risk (The “Mouse Trap”)
The history of longevity research is littered with compounds that worked in mice but failed in humans (e.g., Resveratrol).
-
Sex Specificity: The ITP data showed efficacy only in males. If this sex-specificity holds in humans, it would severely limit the market potential. Adjuvia must elucidate the mechanism of this difference (likely hormonal) and demonstrate efficacy in females in their specific disease models.
-
Dosing: The ITP used a massive dose (4000 ppm). Adjuvia asserts their nanoparticle solves this, but Phase 1 pharmacokinetic data will be the ultimate truth test. Can they achieve “ITP-levels” of astaxanthin in human blood without toxicity?
8.2 Regulatory Hurdles
The FDA does not recognize “aging” as a disease. Adjuvia’s strategy to target “Mitochondrial Disease” is sound, but clinical endpoints in these heterogeneous diseases are notoriously difficult to measure. Demonstrating a functional improvement (e.g., 6-minute walk test) in mitochondrial myopathy patients will be the critical hurdle.
8.3 Competition
There is a “Gold Rush” in mitochondrial therapeutics. Competitors are developing various approaches (e.g., NAD+ precursors, cardiolipin stabilizers like Elamipretide). Adjuvia must prove that ATI-103 is not just an antioxidant, but a superior disease-modifying agent.
9. Conclusion
Adjuvia Therapeutics presents a compelling case for ATI-103 as a first-in-class mitochondrial therapeutic. The company has successfully triangulated its value proposition using three distinct lines of evidence:
-
Evolutionary Biology: The “Salmon Paradigm” and the remarkable longevity of the Clownfish and Batfish provide a strong biological rationale for astaxanthin’s role in stress resistance.
-
Gold-Standard Pharmacology: The ITP’s Harrison et al. (2023) study provides undeniable proof that the astaxanthin molecule can extend mammalian lifespan and delay pathology, validating the core scientific thesis.
-
Technological Innovation: The proprietary nanoparticle platform addresses the historical Achilles’ heel of the molecule—bioavailability—transforming a supplement into a potential drug.
By targeting mitochondrial dysfunction—the nexus of aging and chronic disease—ATI-103 has the potential to transcend the category of “antioxidant” and become a true geroprotector. If the company can successfully navigate the IND process and replicate the “Orby” and “ITP” results in human clinical trials, ATI-103 could represent a breakthrough in the treatment of mitochondrial myopathies and, eventually, the aging process itself.
Recommendation:
For stakeholders in the longevity and biotech space, Adjuvia Therapeutics warrants close observation. The convergence of high-quality NIA validation with a novel delivery system creates a risk/reward profile that is notably superior to typical early-stage longevity biotech ventures.
Statistical Appendix: Data from Harrison et al. (2023)
| Metric |
Astaxanthin (Male) |
Control (Male) |
Result |
| Median Lifespan |
~960 days |
~860 days |
+12% (p<0.001) |
| 90th % Lifespan |
Increased |
Baseline |
Significant |
| Dose |
4000 ppm |
0 ppm |
High Dose |
| Start Age |
12 Months |
12 Months |
Mid-life Intervention |
| Site Consistency |
3/3 Sites |
N/A |
Highly Robust |
Table 1: Summary of lifespan extension data for Astaxanthin in genetically heterogeneous UM-HET3 mice, as reported by the Interventions Testing Program (ITP) in Harrison et al., GeroScience 2023.