I found this interesting
I’ll bet Dr Sinclair claims to solve it by then.
Since aging will not be “solved” in the next 10 years, I’d happily bet against anyone who thinks it will.
What odds do you want to give and how do you define this?
As you know I think I have “solved aging”, but that is by understanding the cause of the main phenotypes of aging.
Rather than a big breakthrough, what if the answer is already before our eyes?
The real catalyst to longevity isn’t a top-down discovery from billion-dollar research, but a bottom-up revolution driven by people like us – informed, proactive individuals experimenting responsibly and proving it works in the real world? Let me explain my take, based on my own journey (I’m 72 now, feeling sharper and more energetic than I did a decade ago).
I’ve “accepted” the (ITP) studies on mice – and take all of their top winners. These aren’t pie-in-the-sky hypotheticals; they’re already showing robust, reproducible effects. Inspired by that, I’ve incorporated most into my regimen.
For the prescriptions, I sought out specialists who understand metabolic health and longevity research. I stack them with over-the-counter stuff backed by ITP. Regular bloodwork keeps me monitoring biomarkers to stay safe.
Here’s the key part: I haven’t looped in my primary care physician (PCP) yet. Why? At this stage, most mainstream docs are conservative – trained to treat diseases, not optimize for longevity. They’d likely wave it off as “unproven” or worry about off-label use. But fast-forward to when I’m 80 or 85, still hiking mountains, crushing cognitive tests, and dodging the usual age-related pitfalls. That’s when I plan to share the full picture: “Hey doc, here’s what I’ve been doing, here’s my data over the years, and look at me now.” If I’m thriving without issues, what can they say? They’ll have to grapple with the evidence right in front of them. And I’m not alone.
As more of us in the longevity community do this, we’ll create a wave. Imagine your PCP seeing 5, then 10, then 50 patients in their 70s and 80s who are outliers in healthspan, all pointing to the same ITP-inspired protocols. Skepticism crumbles when it’s not abstract studies, but living, breathing proof. Docs start prescribing these off-label more confidently. Insurance companies notice lower healthcare costs for these “super-agers” and push for coverage. Regulators follow suit, fast-tracking human trials or approvals based on mounting real-world evidence. This isn’t waiting for the “breakthrough” – it’s being the breakthrough.
Of course, this isn’t medical advice – do your homework, consult pros, and prioritize safety. But the substances are already on the table for middle-aged people to reach escape velocity. I’m glad to be a member of this community that is almost literally paving the way.
I believe what Gries is refering to is not a slowdown of the aging process which can be achieved with current medicine but rather a total stop and potential reversal of aging processes which extend biological lifespan of mammals indefinitely.
Yes, agreed, it is the ultimate ideal goal. The “escape velocity” concept is all that I can realistically hope for, and in practice it would be “good” enough to accomplish the mission, i.e. buy time until the final solution.
I know such a bet depends on the definition of “solving aging”, but even if we use a concervative definition of solving aging merely meaning we can stop it close to 100% but not stop it 100% or reverse it I still think we will not solve it by 2035. I would bet a lot on that, and would love to lose.
This is one of the many reasons I don’t trust your judgement when it comes to the biology of aging. I think anyone that claims to have “solved aging”, even if only hypothetically (in terms of knowing exactly what we need to do to solve it if we just had the technology to do it), is full of themselves and is greatly underestimating the complexity of the problem. There are millions of problems in the world that are orders of magnitude simpler to solve than solving aging but have still not been solved.
Of course a lot of people could claim to have solved aging if their definition of that is simply to identify one of the many different causes of aging. But IMO that’s really just a small first step and nowhere close to solving aging. For many of the problems there are no solutions in sight.
One of the key things for me are the inter-dependencies and how they will affect any “solution” for aging.
Introduction
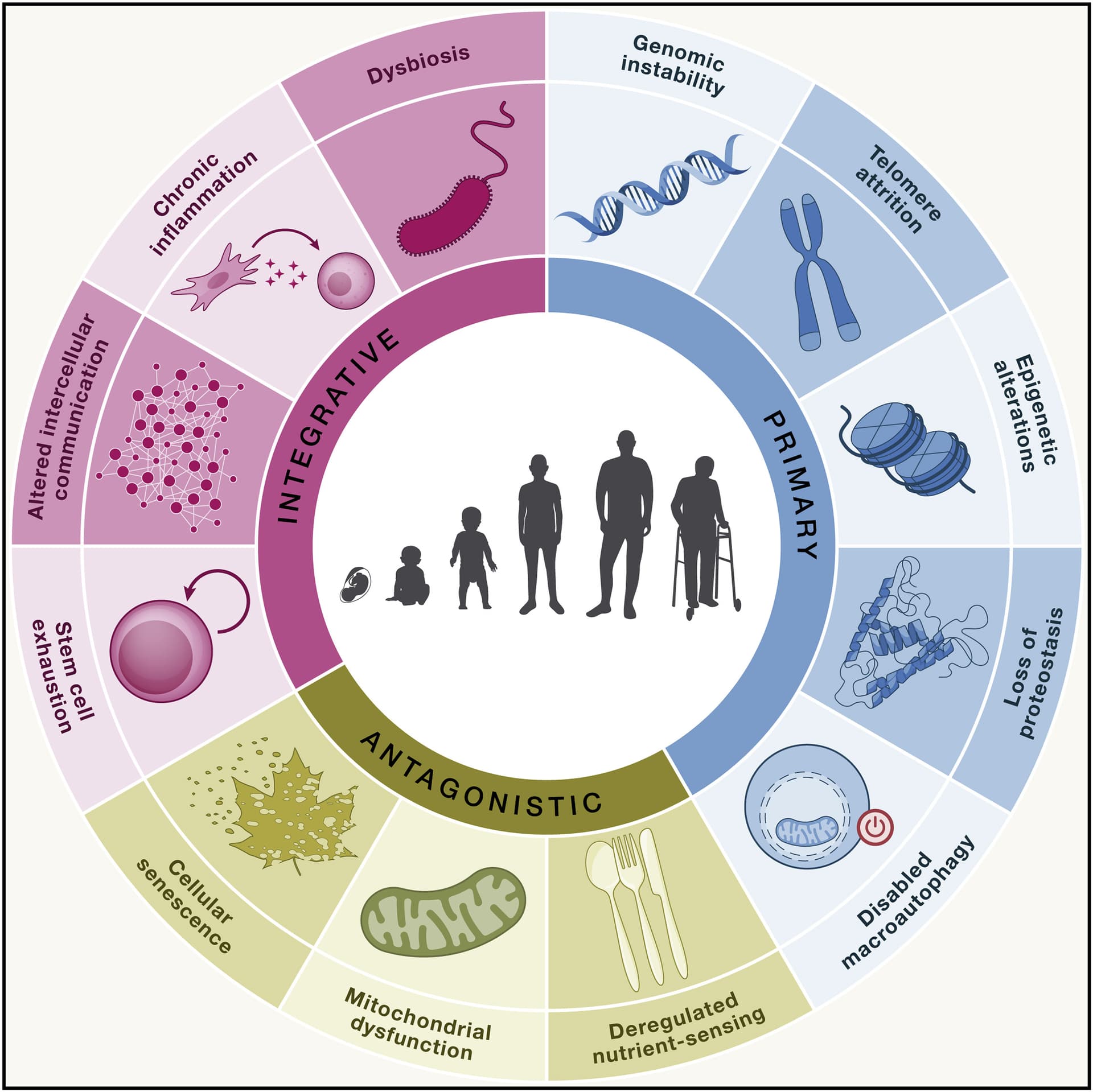
Aging research explores the decline in function of organisms during adulthood. Since 2013, when the first edition of the hallmarks of aging was published in Cell,1 close to 300,000 articles dealing with this subject have been published, which is as many as during the preceding century. Hence, time has become ripe for a new edition of the hallmarks of aging incorporating the main knowledge obtained a decade on.
The distinction among “hallmarks” is intrinsically diffuse, since they interact and are not independent of each other. Therefore, their classification is inevitably arbitrary, but we proposed three criteria that must apply for each hallmark of aging: (1) the time-dependent manifestation of alterations accompanying the aging process, (2) the possibility to accelerate aging by experimentally accentuating the hallmark, and—most decisively—(3) the opportunity to decelerate, halt, or reverse aging by therapeutic interventions on the hallmark.
https://www.sciencedirect.com/science/article/pii/S0092867422013770
Examples of Interdependencies
Malfunctioning mitochondria produce more reactive oxygen species (ROS), which are known to damage DNA, leading to genomic instability.
Senescent cells secrete inflammatory factors, a process known as the senescence-associated secretory phenotype (SASP), which drives chronic inflammation.
A declining number of stem cells, combined with inflammatory signals from the aging microenvironment, reduces their ability to differentiate and repair tissues.
- Dysbiosis and Chronic Inflammation:
An imbalanced gut microbiome (dysbiosis) can trigger and amplify inflammatory responses, contributing to systemic inflammation that is a hallmark of aging.
Impaired protein quality control (proteostasis) can lead to the accumulation of damaged proteins, some of which may be mitochondrial proteins, further contributing to mitochondrial dysfunction.
Implications for Research and Therapy
- Complexity:
Understanding these interdependencies is crucial because aging is a complex biological process with multiple interacting molecular pathways.
- Therapeutic Targets:
New potential anti-aging strategies could be developed by targeting specific interactions, such as how mitochondrial dysfunction contributes to other hallmarks.
- Holistic Approaches:
Interventions that consider aging as a whole, rather than focusing on individual hallmarks in isolation, may be more effective in modifying the rate of aging.
- Expanding the Framework:
New hallmarks are continually being added, and the concept of “meta-hallmarks” helps to integrate common foundations of aging and cancer, further emphasizing the interconnectedness of these processes.
The hall marks are all affected by gene expression. There was a paper earlier this year that went through a number of species linking hall marks to mtDNA damage.
“fixing” mtDNA is not going to extend human life span but should significantly increase health span. And that is all I care about today ![]()
I’m going to be trying a couple things that I’ve been putting off. I just took my first 4.0mg dose of ARA 290 a couple hours ago and will do a once a day cycle for 30 days, when I finish that I’ll be doing a 1 month course of ASHA-091
ARA 290
ARA 290 can help protect mitochondrial DNA (mtDNA) indirectly by reducing DNA damage and oxidative stress. Studies show that ARA 290 acts as a cytoprotective agent that significantly reduces genotoxicity caused by agents like doxorubicin (a chemotherapy drug) by:
-
Attenuating oxidative stress and reactive oxygen species (ROS) generation, which are major contributors to mtDNA damage.
-
Enhancing antioxidant enzyme activities such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), which protect cells from oxidative damage. -
Reducing inflammation and apoptotic cell death that otherwise exacerbate DNA damage. -
Displaying genoprotective effects in different cell types under toxic stress conditions. -
While not directly repairing mtDNA, ARA 290’s anti-inflammatory, antioxidant, and cytoprotective effects create a cellular environment supportive of mtDNA integrity and protection against damage.
ASHA-091 a DRP1 inhibitor that affects mitochondrial DNA (mtDNA) integrity in several key ways:
-
DRP1 mediates mitochondrial fission, a process essential for maintaining mitochondrial quality control and distribution, but excessive or dysregulated DRP1 activity leads to excessive mitochondrial fragmentation.
-
Excessive mitochondrial fragmentation disrupts mitochondrial networks, leading to impaired mitochondrial function, increased reactive oxygen species (ROS), and damage to mtDNA. -
Inhibition of DRP1 reduces mitochondrial fragmentation, resulting in longer and more interconnected mitochondria with improved membrane potential and reduced ROS production. -
By preserving mitochondrial morphology and reducing oxidative stress, DRP1 inhibition helps maintain mtDNA integrity and reduces mitochondrial dysfunction-linked cell death. -
DRP1 inhibition has been shown to protect against mitochondrial damage caused by stressors like radiation and neurodegenerative stimuli by preventing excessive fission-induced mtDNA and mitochondrial membrane damage. -
However, DRP1-mediated fission is also important for proper mitochondrial distribution during cell division and mitophagy; thus, complete loss of DRP1 can impair normal mitochondrial turnover.
It would be useful to see the train of evidence to those via links.
I worry about how people view fission.
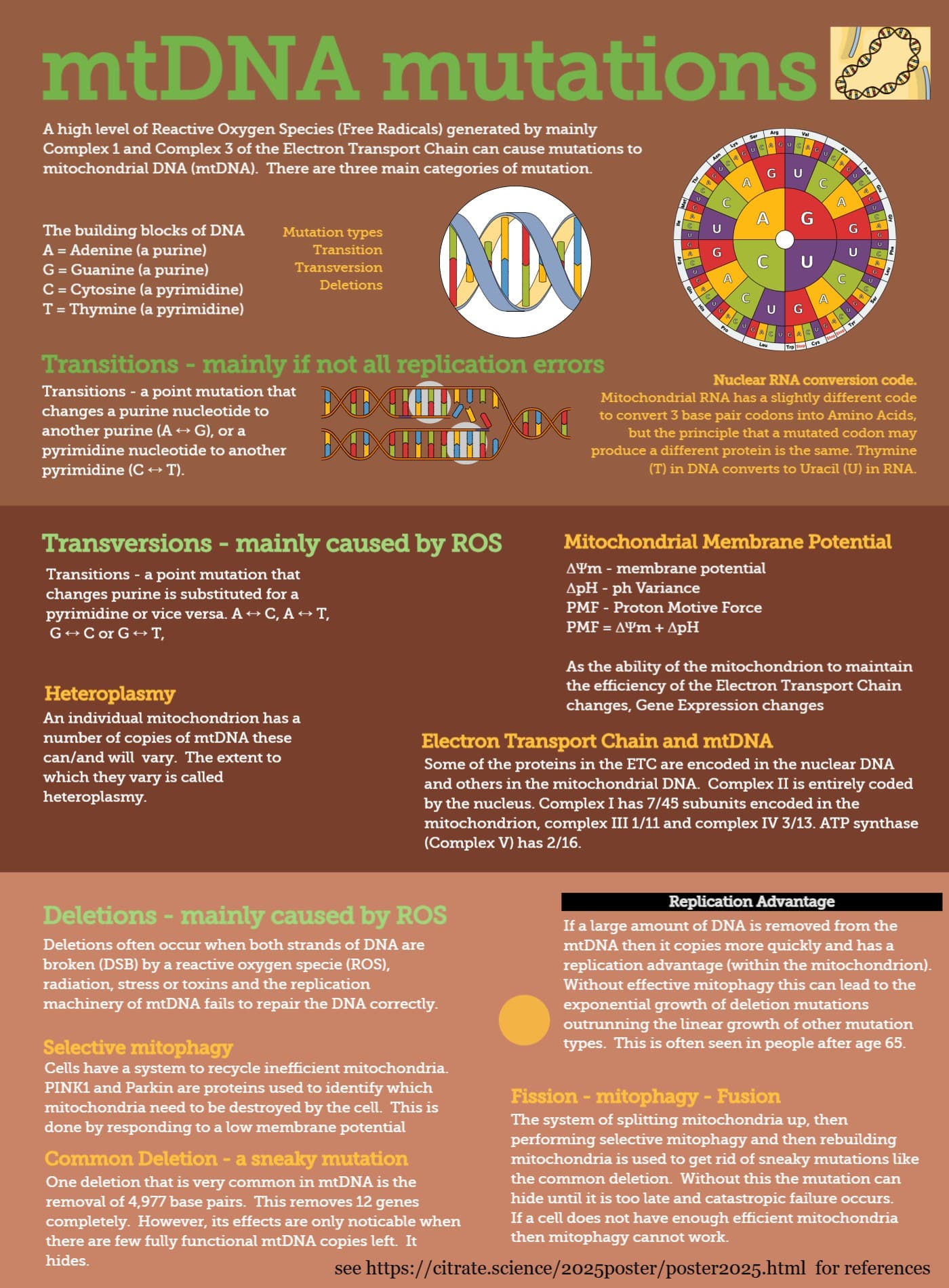
Certain mtDNA mutations hide. It is particularly the deletions that hide as they cause ETC proteins to not be produced. However, as mitochondria can produce the required proteins from any available mtDNA ring and there are multiple rings in each mitochondrion the mitochondria can continue functioning until the proportion of mtDNA with deletions hits a high threshold (between 60 and 80%).
To find those and remove them there is the fission-mitophagy-fusion process where the mitochondria are chopped up so that those which have the bad mtDNA in them are then easy to identify as their mmp drops.
If there are lots of fragmented mitochondria that is more likely to be cause the mitophagy process has not completed properly than there is too much fission. Hence preventing fission may temporarily improve cellular function, but create catastrophic and unpreventable failure at a later stage.