https://iovs.arvojournals.org/article.aspx?articleid=2127832
Table 1
Age, SE, axial length and 360° RNFL thickness in EM, LM, MM and HM groups
| Variables | EM | LM | MM | HM |
|---|---|---|---|---|
| Age (y) | 31.85±10.55 | 31.10±9.64 | 31.38±11.03 | 31.15±8.68 |
| SE (D) | -0.11±0.21 | -1.47±0.73 | -4.45±0.81 | -8.25±1.41 |
| Axial length (mm) | 23.18±0.75 | 23.74±0.70 | 25.03±0.85 | 26.37±1.35 |
| Average RNFL thickness (µm) | 100.91±10.07 | 98.68±8.81 | 94.97±9.02 | 89.88±9.41 |
=> this is why it’s important
There was a statistically significant difference in the average RNFL thickness between the three degrees of myopia groups, with least myopia having higher RNFL thickness and high myopia with least RNFL thickness (P = 0.001); low myopia group mean RNFL being 92.17 (+9.84), moderate myopia group 88.12 (+9.53) and high myopia group 82.40 (+10.
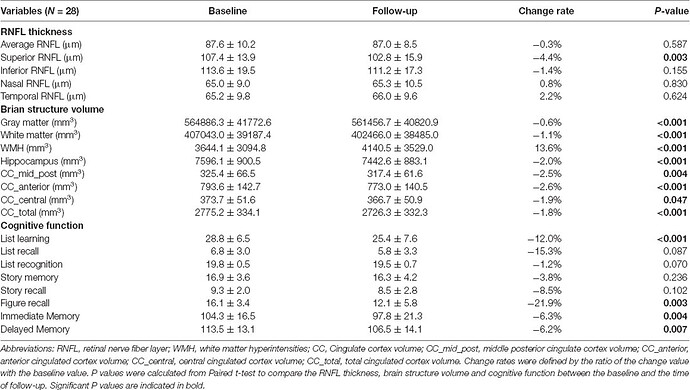
Recently, two population-based cohort studies further reported that thinner RNFL was associated with current and future cognitive impairment, and could predict the incidence of dementia within 4.5 years in nondemented participants (Ko et al., 2018; Mutlu et al., 2018). These findings suggested that retinal and brain neurodegeneration may occur in parallel to some extent, and RNFL thickness could serve as a noninvasive and less expensive biomarker of AD.
Given the wide variance of RNFL thickness in old adults, OCT measurement at a single time point may not be informative enough to the identification of abnormal RNFL profiles in AD patients (Mok et al., 2002). In our previous studies, we found that longitudinal reduction of RNFL thickness was associated with the decline of episodic memory in old adults (Shen et al., 2013), and those who suffered cognitive decline within 25 months had more reduction of RNFL thickness with respect to old adults with stable cognition (Shi et al., 2014, 2016). It supports the hypothesis that a longitudinal change of RNFL thickness would be more indicative of cognitive deterioration among the elderly population