I’m on a low salt diet. When I cook for myself I never add salt. I’m used to natural taste of food without salt. I get some salt in breads and cheese that I buy. My sodium level per the most recent labs was 135 pushing toward the lower limit of normal. I’m wondering if it could be too low.
Just got my numbers back today. Normal range for sodium 135-146mmol/L. You’re still in there. I use salt on meat and eggs, eat a lot of cheese, my number was 140.
That’s what mine was on my last labs and I’m personally fine with that result. My doctor is as well fwiw.
Big Food vs. Big Pharma.
![]() Can’t stop eating too much
Can’t stop eating too much ![]() GLP-1/GIP agonist
GLP-1/GIP agonist
![]() Can’t stop eating too much sugar
Can’t stop eating too much sugar ![]() SGLT2 inhibitor
SGLT2 inhibitor
![]() Can’t stop eating too much saturated fat
Can’t stop eating too much saturated fat ![]() HMGCR inhibitor
HMGCR inhibitor
![]() Can’t stop eating too much salt
Can’t stop eating too much salt ![]() NHE3 inhbitor?
NHE3 inhbitor?
2014:
The researchers gave the experimental drug to dozens of healthy people for seven days and gave others a placebo. Tenapanor increased the amount of sodium exiting in feces and reduced sodium excreted in urine, indicating that volunteers on the experimental drug absorbed less salt and processed less through their kidneys. Tenapanor itself didn’t show up in the blood of most people taking it, suggesting the compound often goes no farther than the intestines. That’s important because sodium plays fundamental roles in the body and wiping it out elsewhere would be dangerous, Zachos says. No adverse effects were reported in the human tests.
https://www.sciencenews.org/article/experimental-drug-might-get-salt-out
SGLT2 inhibitors mentioned affecting interstitial storage of sodium.
Although interstitial storage of sodium is a new, and for many clinicians unknown concept, it is likely that most clinicians have actively altered interstitial sodium content in their patients last month. Data from 23Na-MRI studies show that everyday treatments such as diuretics, sodium glucose cotransporter 2 (SGLT-2) inhibition and dialysis significantly impact interstitial sodium content [17, 18, 43]. These therapies may be of particular interest in sodium-sensitive hypertension as these subjects are characterized by interstitial sodium accumulation. Until recently, it was unknown whether interstitial sodium accumulation contributes to the cardiovascular risk. Yet, a recent trial demonstrated an association between skin sodium content and an intermediate endpoint, left ventricular hypertrophy. In chronic kidney disease patients, interstitial sodium content was strongly correlated with left ventricular mass, independent of blood pressure or total body overhydration [21]. The observed correlation was stronger than the correlation between total body overhydration and left ventricular mass. Although we need to wait for data from long-term studies investigating the potential cardiovascular risk that is associated with interstitial sodium accumulation, these data are interesting, in particular because interventions are possible with commonly used therapies.
Longitudinal studies evaluating interstitial sodium in children and adolescents using methods such as 23Na-MRI could reveal the direction of causality in the relationship between interstitial sodium and blood pressure, showing if interstitial sodium accumulation starts early in life and precedes, or is implicated in the development of hypertension. This may further strengthen the need for preventative strategies such as dietary sodium reduction in children to prevent the onset of hypertension in adulthood.
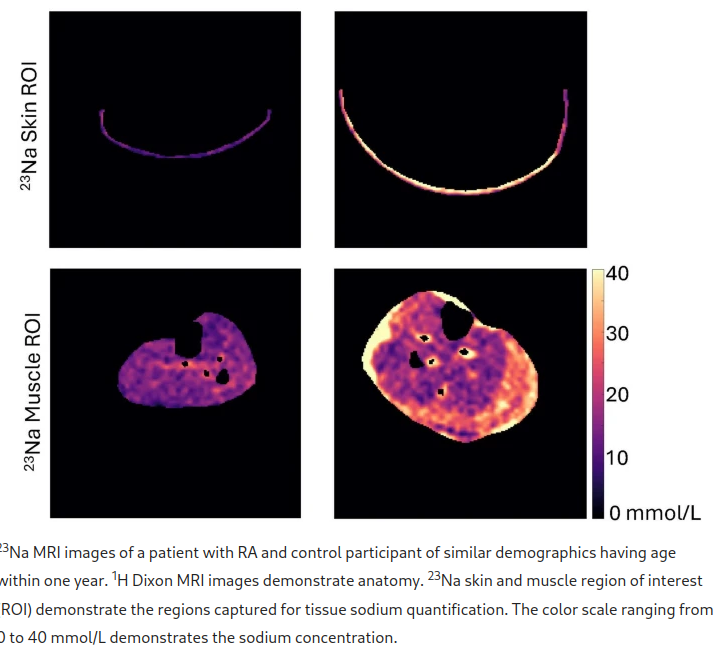
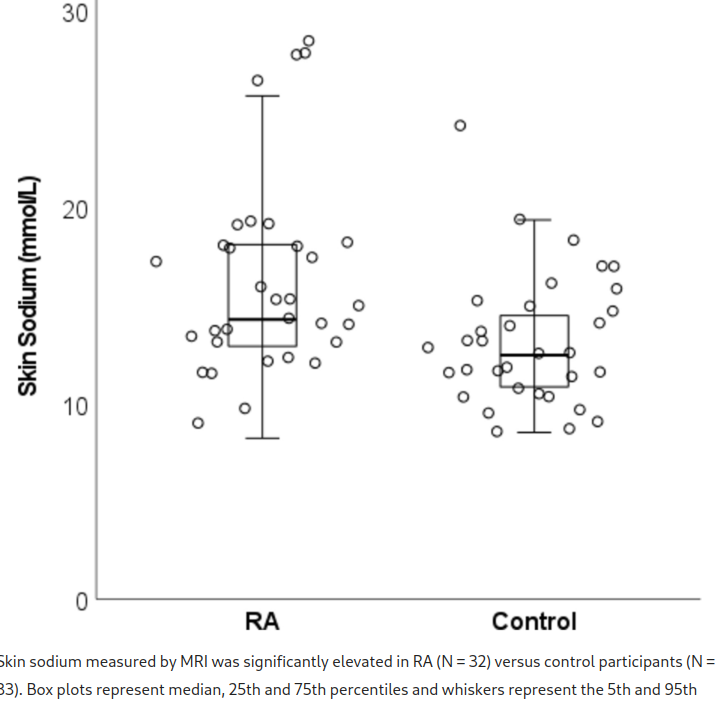
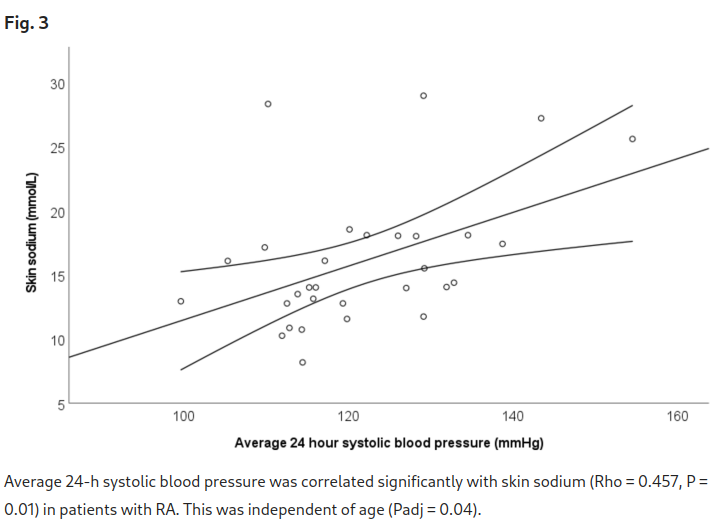
Patients with rheumatoid arthritis (RA) have increased hypertension. Tissue sodium may contribute to development and progression of hypertension through immune cell activation. This study aimed to determine if skin sodium content is: 1) higher in RA versus control participants, and 2) associated with blood pressure and disease activity. This cross-sectional study included 32 patients with RA and 33 control participants. Lower leg skin sodium content was measured using magnetic resonance imaging. Ambulatory 24-h blood pressure measurements were obtained, and disease activity was assessed by Disease Activity Score-28 for RA with CRP (DAS28-CRP). Skin sodium content was higher in RA versus control participants (14.22 [12.82, 18.04] vs 12.41 [10.67, 14.55] mmol/L), p = 0.005. Every 1 mmol/l increase in skin sodium was associated with a 1.05 mmHg (95% CI 0.29, 1.82 mmHg, p = 0.009) increase in average 24-h systolic blood pressure in patients with RA, but this relationship was not present in control participants. Skin sodium was not associated with DAS28-CRP or its components. Skin sodium is increased in RA versus control participants and is correlated with 24-h and diurnal systolic blood pressure in patients with RA but not in control participants. Skin sodium content may help explain increased hypertension in patients with RA.
For many years, excess sodium was thought to cause hypertension solely through its osmotic effect in the intravascular space33. However, sodium accumulates in the extracellular matrix (ECM) of the skin in particular, and in other tissues such as muscle34. The result is a reservoir of osmotically inactive, but immune-active sodium35. Monocytes and macrophages migrate to sites of higher salt concentration where they take in the sodium from the interstitium36. The sodium-laden macrophages are proinflammatory and promote vasoconstriction and sodium retention37. These findings have led to a paradigm shift, in which the accepted theory in electrolyte and fluid physiology seems an oversimplification and the relationship between dietary sodium intake and blood pressure is more intricate than was believed33,38.
Nature, Dec, 2024.
https://www.nature.com/articles/s41598-024-83873-8
Regulation of sodium ion channels in the heart and kidneys may also be a way in which SGLT2i produce their cardiorenal benefits. For example, evidence suggests that dapagliflozin may help to reduce blood pressure by improving the sodium balance [90]. The sodium-hydrogen exchanger-3 (NHE3) in kidneys mediates sodium reabsorption following renal glomerular filtration [91,92,93]. Reduced NHE3 activity in the proximal tubule following treatment with SGLT2i leads to decreased sodium reabsorption followed by lowering of glomerular pressure, and reduction of blood and tissue fluid volume. Sodium-hydrogen exchanger-1 (NHE1) is also thought to play an important role in the pathogenesis of HF [91]. NHE1 activity is raised in HF, and its inhibition decreases susceptibility to severe ventricular arrhythmia, reduces contractile dysfunction and limits tissue death/damage both when blood supply to the heart muscle is insufficient (ischaemia), and when it subsequently returns (reperfuses) [94]. Benefits of SGLT2i on HF have been suggested to be mediated by inhibition of sodium-hydrogen exchange in the heart via NHE1, thus resulting in reduced sodium concentration [14, 42, 95,96,97]. It is possible that SGLT2i may favourably induce cardiac remodelling by these mechanisms, thus reducing the risk of arrhythmias and sudden death [42, 97]. However, a recent study found that empagliflozin and other SGLT2i had no effect on cardiac NHE1 activity over a range of concentrations, including the therapeutic dose, warranting further research on this hypothesis [98].
No Na intake does not only increase blood volume with subsequent hydration, this is not what the newest science show. It’s stored in tissues in the body and being inactivated before storage. The sodium stores in the skin might e.g affect BP by causing vasoconstriction, at least maybe in autoimmune groups.
spironolactone could help too
I’ll have to see a trial if it affects tissue sodium, saw a trial for e.g. dapagliflozin, and empagliflozin.
I’m getting a bit convinced that tissue sodium storage is a psuedo hallmark of aging.
Accumulates in tissues, causing damage, involved in many different diseases states, prevention or reducing should or could be relatively low risk (which is what is wanted from a longevity drug). Isn’t related to hydration or dehydration per se (although dehydration causes aging in mice).
We need a graph of tissue sodium on a MRI with different age groups I think. For now we know the MRI lights up at 85 yrs old + hypertension, compared with health 20 something old, for example.
Thank you @A_User for this goldmine of information. It is unbelievable how little we really understand about the impact of sodium over a lifetime. I’d personally love to have a Na MRI done and would gladly participate in any future studies.
Yes this new salt paradigm is really interesting. It’s great that a YouTuber like Goobie has presented this information to a wider audience. By the way, thank you @A_User for pointing me to him. He is a nice and very bright guy.
I found this site by accident when I was searching for a new dietary sodium to potassium ratio calculator because the one I had been using was down. It’s not a calculator but I found it pretty interesting. It discusses many of the things already covered in this thread like the Mars study, sodium being stored in the skin, the Yanomami people etc but then seems to have died in around 2016. I’m not sure why…everything is still online but there’s nothing new posted as far as I can tell. Perhaps there’s nothing new to add. Some of it could be quackery and I apologize if so. I don’t feel qualified to judge if everything on the site is correct tbh. Here it is though for anyone curious.
I’m wondering if anyone here currently eating a no added salt diet is also taking dapagliflozin and if so how it is going? Thanks
Prevalence of Admission Hyponatremia in Patients With Diabetes Treated With and Without an SGLT2 inhibitor
https://academic.oup.com/jes/article/7/4/bvad011/6998591
“Hyponatremia often reflects a free water excess. Sodium/glucose cotransporter 2 (SGLT2) inhibitors increase free water excretion through glucose-induced osmotic diuresis. In 2 randomized double-blind, placebo-controlled trials in patients with the syndrome of inappropriate antidiuresis (SIAD), we showed that empagliflozin increased plasma sodium concentration more effectively than placebo.”
“The main finding of this cross-sectional study is that hyponatremia prevalence and plasma sodium concentration were the same in patients with T2DM treated with and without SGLT2 inhibitors, irrespective of comorbidities and comedications.”
“Second, the inhibition of SGLT2 increases glucosuria and natriuresis [38]. One could argue that it would increase urinary sodium clearance and worsen hyponatremia. However, hyponatremia is not a side effect of SGLT2 inhibitors, mainly because the pathophysiology of hyponatremia relies more on a relative water excess than an absolute sodium deficit [39]. Interestingly, our data showed no difference in urine sodium concentration and fractional excretion of sodium between patients with SIAD treated with empagliflozin or a placebo [25, 26]. In patients with T2DM, natriuresis seems to be transient as well [40].”
Severe hypernatremia caused by diabetes drug – a case study report
“Irrespective of severity, hypernatremia may be caused by salt (sodium) overload but is most commonly due to water deficit (i.e. dehydration). This recently published case study report highlights severe hypernatremia due to water deficit. In this case water deficit was attributed to the blood-glucose-lowering drug empagliflozin that is used to help normalize the blood glucose concentration of patients with type 2 diabetes.”
Thank you kindly for the helpful links Cronos.
I did find one study from 2021 comparing dapagliflozin in high sodium v low sodium participants in the context of DKD fwiw. I’m not qualified to judge the quality of the study but figured I’d share here if anyone has an interest in reading it.
https://www.nature.com/articles/s41598-020-79687-z
I’m not clear what qualifies for LS in their study but I suspect it’s still much higher than I consume.
Not the exact answer you are looking for, but I take dapagliflozin, and, until last week, on many days I’d only consume 1/4 tsp-ish of salt that was used in cooking.
On the days I eat shelf stable products, like RAO’s sauce, it would obviously be a lot higher.
I don’t notice any differences either way, fwiw.
That’s actually very, very helpful Beth! Thank you! 1/4 tsp of salt is equivalent to about how much I get from the naturally occurring sodium my no added salt omnivore diet. I’m mostly interested in seeing if it helps me with reactive hypoglycemia as it has others here. If I could fix that issue it would truly be life changing.
Dr. Hashmi chimes in on fluid intake, sodium and kidney health. In the notes on the video he lists all the studies he references.
AI gives this summary (by mistake I just submitted the transcript)
Quick-look takeaway
- Healthy adults usually do best with ~2 – 3 L of total drinking water a day (all beverages counted). That amount aligns with the U.S. National Academies’ Adequate Intake targets once the ±20 % water that comes from food is subtracted.National Academies Press
- The popular “8 × 8” rule (1.9 L) was never evidence-based; its origin was a mis-read 1945 Food & Nutrition Board statement.PubMed
- More isn’t better: the kidney can clear only ≈0.8–1 L per hour. Repeatedly drinking well above that (≈8 L + spread over a day) can dilute blood sodium below 135 mmol/L (hyponatraemia) and, in extreme cases, cause seizures or death.PubMedNEJM Evidence
- Kidney-stone prevention: ensuring ≥2 L of urine output—roughly 2.5 L + fluid intake for most people—cuts stone recurrence risk about 50 %. Every extra 0.5 L you drink lowers risk another ~7 % up to about 3 L/day.PubMedPubMed
- Existing chronic kidney disease (eGFR < 45 mL/min/1.73 m², “stage 3B” or worse) usually requires individualised restriction to ≤1.5 L/day, sometimes less, to avoid fluid overload.KDIGOPubMed
Thanks @CronosTempi and @John_Hemming. I watched the video and looked in cronometer where I (obsessively ![]() ) track everything that enters my mouth and I’m coming in at about 3 liters per day between all beverages and food. My last sodium level on my blood work was 139 so that’s good. I’ll just keep doing what I’m doing. The AI summary is appreciated as well for easy reference!
) track everything that enters my mouth and I’m coming in at about 3 liters per day between all beverages and food. My last sodium level on my blood work was 139 so that’s good. I’ll just keep doing what I’m doing. The AI summary is appreciated as well for easy reference!