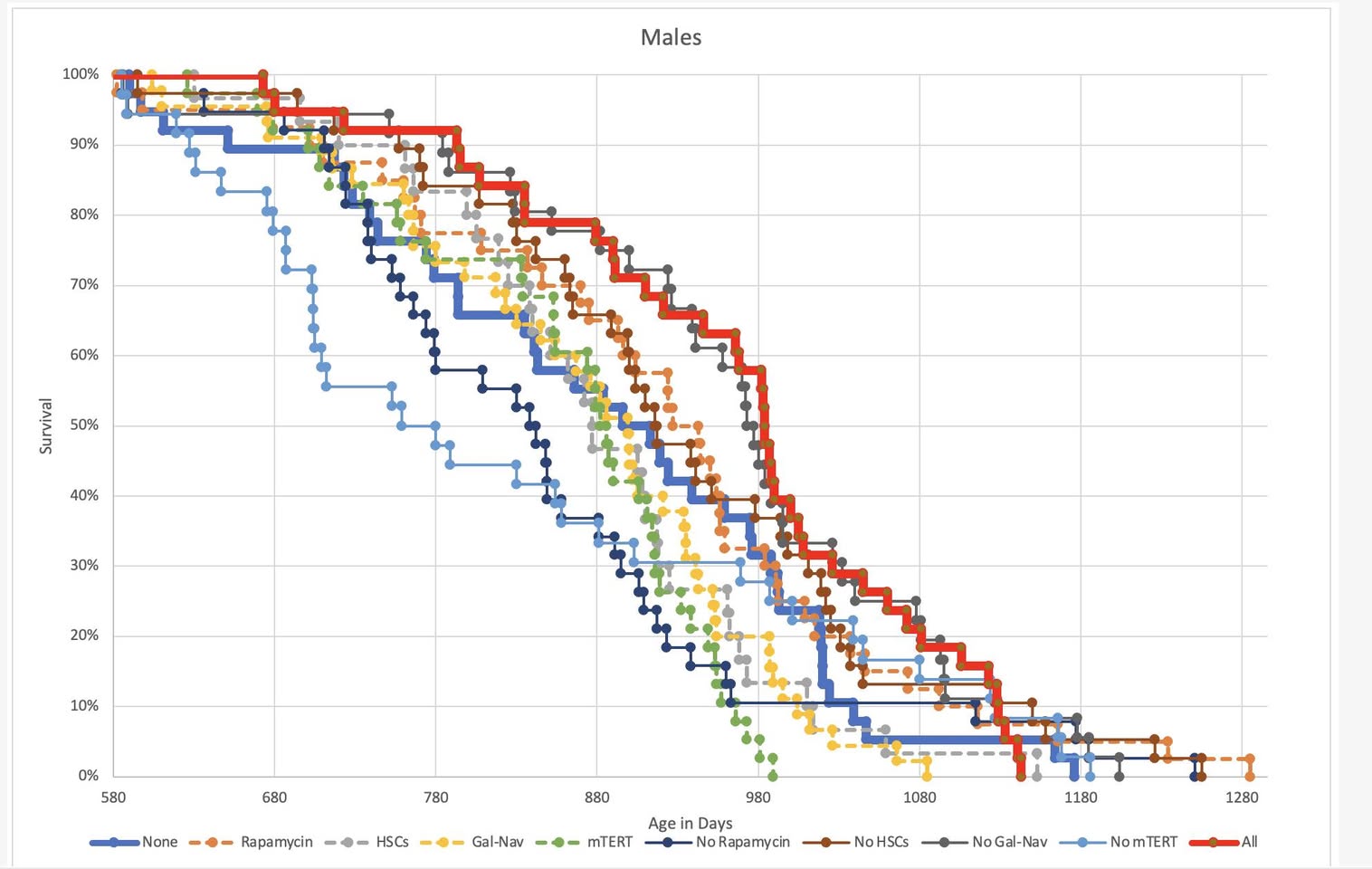
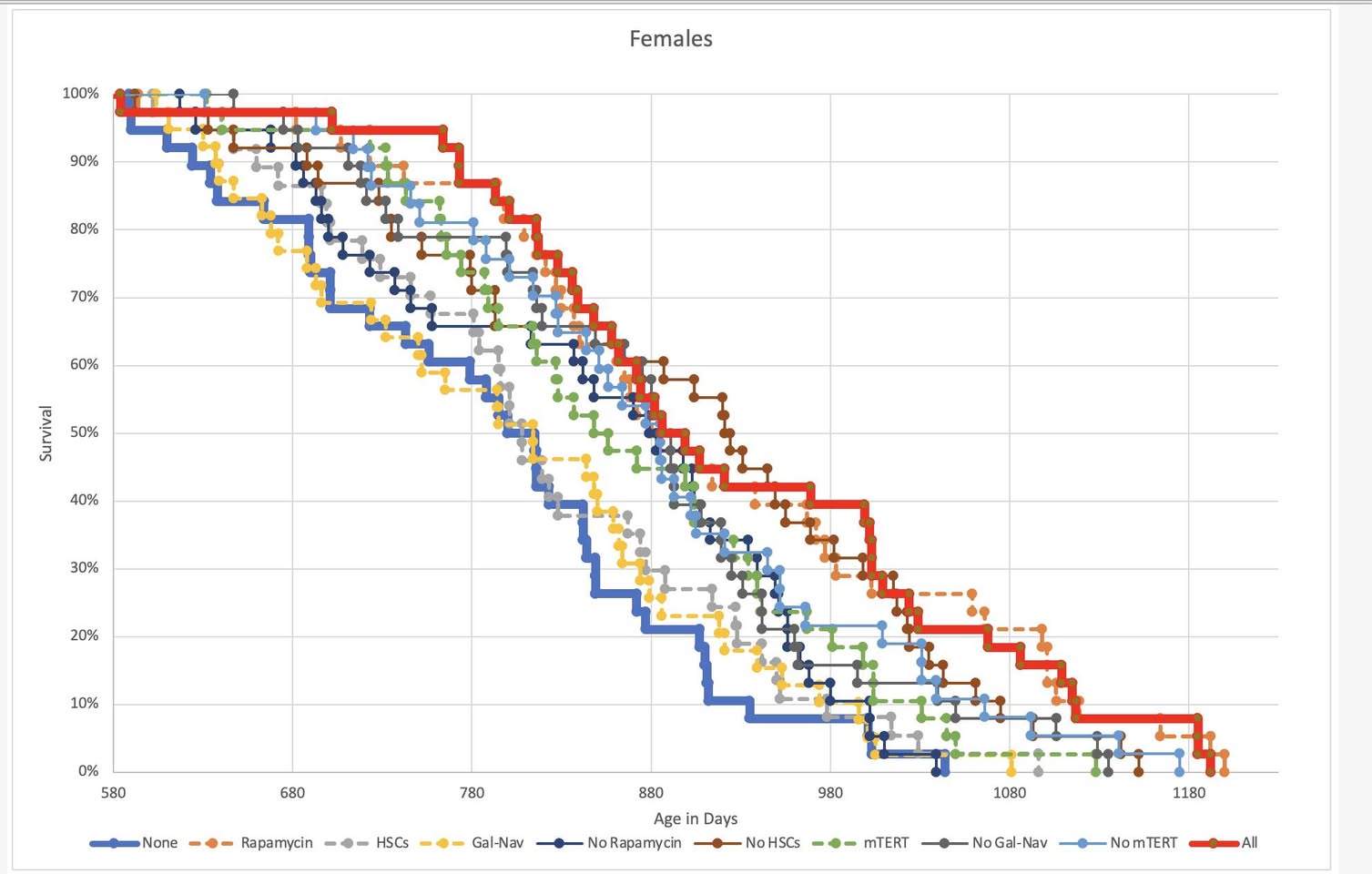
You can contact Aubrey de Grey by just mailing him or just contacting him through his social media. He usually answers. On this page the following is said about the interventions. Please share the answer you get from him here if you get one 
Hematopoietic Stem Cell Transplant
Rationale: A reduced regenerative capacity of stem cells is widely believed to contribute to age-related morbidity and functional tissue decline. Numerous studies have shown stem cell transplantation to have rejuvenating effects in mouse tissues, and several additionally show a lifespan extension effect [PMID 32012439, 31031800].
Design: The study design is largely (but see below) based on the protocol utilized by Guderyon et al. 2020 [PMID 32012439] and consists of mobilizing the recipient bone marrow niche followed by transplant of lineage-depleted hematopoietic stem cells (HSCT).
Bone Marrow: We have opted to use lineage-depleted bone marrow HSCs as opposed to additional selection for and expansion of long-term self-renewing HSCs. This was on the basis that 1) prior lifespan studies used whole or lineage-depleted bone marrow, which may include MSCs or other beneficial cells promoting engraftment, and 2) expansion protocols have not been extensively validated.
Mobilization Protocol: We will follow the mobilization protocol for recipient mice as outlined in Guderyon 2020, consisting of G-CSF + AMD3100. Although other newer mobilization reagents require fewer injections, efficacy is not well established; the current protocol is most common for chemical mobilization, has been used in aged mice, and remains the current standard of care for mobilizing HSCs from human BM donors.
# of cells: Each administration will consist of ~2x10⁷ HSCs, derived from 8 sex-matched C57Bl/6J PEP-BOY (CD45.2) donor mice per injection. This number is four-fold greater than the Guderyon study, however in line with other HSCT studies [PMID 31031800, 18491294]. Additionally, it is demonstrated that % donor cell engraftment is largely based on competition with mobilized recipient cells; thus, increasing the donor to recipient ratio in the blood may allow for increased engraftment with each administration allowing for fewer transplants overall and thus less stress on the animals.
Number of Transplant Injections: While the lifespan cohort of the Guderyon study received a total of 8 HSCTs, each round administered only 5x10⁶ lineage-negative (long-term repopulating) donor cells. We wish to minimize the number of administrations for each mouse. Therefore, we will perform 2 rounds of HSCT, a month apart, and then assess the % donor-derived cells (CD45.2) in peripheral blood after 4-8 weeks (timing to coincide with planned blood draw). Whether or not additional rounds of HSCT are performed (for a given experimental group) will be decided based on percentage engraftment in recipient mice.
Senescent Cell Ablation
Rationale: Senescent cells (SnC) are shown to accumulate with age in nearly all tissues, and multiple studies have now shown improvement in healthspan parameters upon SnC removal. Although few of these studies emphasize lifespan effects, we hypothesize that SnC removal is important for mouse longevity due to the role played in immune decline and in cancer – the two leading causes of death in C57Bl/6J mice. We believe an effective senolytic may not only reduce cancer incidence by removal of cells which are cancer-capable (via senescence escape), but also by improving local immune surveillance against abnormal cells, by reducing the SASP’s cloaking effect and relieving systemic immune fatigue from SASP-driven chronic inflammation. In this way, it is also possible that SnC removal can reduce the age-related increase in susceptibility to pathogens.
Design: Discovery of effective senotherapeutic compounds remains an active area of research, with many promising drug candidates in development alongside approaches such as senolytic vaccination using CAR-T cells or conditional suicide gene therapy. Though these second-generation senolytic therapies likely have much to offer in terms of improved specificity and SnC subtype targeting, we felt the current study would benefit most from the extensive data already collected using first generation drugs, and thus selected the broad-spectrum senolytic Navitoclax for inclusion in RMR1. While navitoclax is generally effective as a pan-senolytic compound, ablating SnCs in multiple tissue types while sparing most normal calls, platelets are particularly sensitive to its mechanism of action, Bcl-xl inhibition. To minimize this undesirable side effect, we employed a galactose conjugation prodrug strategy previously developed by colleagues in the field, wherein a galactose moiety attached to the Navitoclcax molecule renders the drug inactive until it can be cleaved by lysosomal beta-galactosidase, an enzyme consistently and considerably elevated in SnCs. ‘Off-target’ activity, for example, against platelets, is greatly reduced in this way, while efficacy against target SnC populations remains largely intact.