The treated mice first have to reach 20 months old before they can apply the treatment, so the ones they choose all made it to 20 months. Seems like the controls should also be selected to first reach 20 months though as well… but the second plot in the paper in that group does that.
I would suggest that the decision as to which mice are in which group should be made really early and we should see a similar lifespan curve up to the intervention. Otherwise there is sign of a selection bias
I am not really bothered about this paper either way, but if you are referring to another chart please upload it and tell us what is in each line.
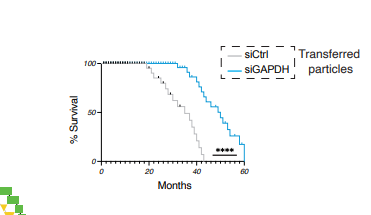
Blue line is with artificial rivers and GAPDH depleted and they grey (control) apparently only with GAPDH in the vesicles. The previous plot was apparently using vesicles which were not artificial - using a donor. So it seems this blue line plot mimics how it would be done in practice, using the artificial approach.
How many mice are in each group?
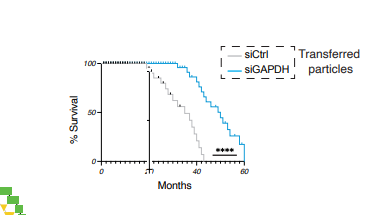
Looking carefully at that image it also has the selection bias issue.
However, I think the question as to how many mice there are in each group also needs resolving.
so they inject a chemical into the blood? or use AAV? or use MRNA?
what do they do exactly? its too deep for me i cant understand
wow rapa and glynac only extends mouse median lifespan by 30%
this guy is doing a 100% extension
I am raising a question about the statistics in the paper.
here is his website Platform - Sentcell®
i dont exactly know fully how it works but i get the idea he is targeting the diseased t cells and then using DOS or whatever its called to rejuvenate them and to remove viruses such as HIV from them and once that is done, the t cells fight HIV, etc themselves and also possibly leads to lifespan extension.
even as a layperson, i do know tcells is quite mainstream in treating cancer and there is a lot of promise in it.
i have no idea how that is done but it seems to be via an injection of some sort.
if you know pls explain to me.
he is curing HIV as well and possibly cancer:
I have asked three LLMs to “do a formal academic review of the attached paper”
chatGPT:
Formal academic review (preprint)
Citation and scope
Manuscript: CD4⁺ T cells confer transplantable rejuvenation via Rivers of telomeres (bioRxiv preprint posted Nov 16, 2025).
This review evaluates conceptual novelty, evidentiary support, methodological rigor, statistical interpretation, and translational plausibility.
Summary of aims, approach, and principal findings
The authors propose a systemic “rejuvenation” mechanism in which CD4⁺ T cells, after acquiring telomere-containing vesicles from antigen-presenting cells (APCs), generate and release extracellular “telomere Rivers”—structured telomeric particle networks that circulate and purportedly reverse tissue senescence across organs.
Key mechanistic claims are that River formation requires:
- Fatty acid oxidation (FAO) via CPT1A, acting through a ceramide/ceramidase → phosphatidylethanolamine axis to restore synapse function and telomere acquisition in senescent CD4⁺ T cells.
- Asymmetric division–like segregation in telomere-recipient CD4⁺ subsets, producing particles that are GAPDH-low/negative and enriched in “stemness” factors.
- Exosome/vesicle secretion pathways (e.g., GW4869 sensitivity) and dependence on antigen-driven APC–CD4 interactions (anti-LFA-1 blockade; CD4 depletion).
Functionally, the authors report:
- Reduced senescence markers (β-gal activity, p16^INK4a, IL-6, “sMAC”) and increased tissue telomere length after River transplantation.
- Large lifespan effects: transplant of ~5,000 sorted River particles into old mice allegedly extends median lifespan by ~17 months, with some animals living to ~58 months.
- “Artificial Rivers” created by silencing GAPDH in APCs phenocopy natural Rivers and may be more potent.
- Presence of analogous particles in human plasma and plant xylem, argued to support deep evolutionary conservation.
Significance and novelty
If validated, the work would be high impact: a transplantable, particle-mediated systemic rejuvenation program linked to adaptive immunity and telomere biology, with a claimed magnitude of lifespan extension rarely seen outside a narrow set of robust geroprotective interventions. The mechanistic chain (APC → CD4 telomere acquisition → CD4-derived secondary particles with altered proteome) is also conceptually original and goes beyond classic “young blood factor” framings.
However, the extraordinary magnitude of the reported survival effects substantially raises the bar for methodological transparency, replication, and exclusion of confounds.
Major strengths
-
Mechanistic perturbation across multiple nodes
- CPT1A restoration in senescent CD4 T cells, lipid/ceramide pathway interventions, asymmetric-division disruption (anti-LFA-1), secretion inhibition (GW4869), and timing experiments with cytochalasin D provide a reasonably dense causal narrative.
-
Attempted compositional validation
- Proteomics (DIA/DDA) and targeted assays emphasize GAPDH depletion and enrichment of candidate “stemness” factors in Rivers vs APC vesicles.
-
In vivo dependency controls
- River production dependence on antigen-pulsed APCs, CD4 sufficiency, and asymmetric-division blockade is a strong design principle for establishing physiological regulation.
-
Operational definitions for sorting
- Rivers defined as PKH67⁺ TelC⁺ GAPDH⁻ particles; authors state high sorting purity (~97%) and standardized serum input volumes in at least some contexts.
Major concerns (must be addressed)
1. Plausibility and documentation of the lifespan phenotype
A reported shift of ~17 months in median lifespan, with some mice reaching ~58 months, is exceptionally large for standard laboratory strains and demands maximal transparency.
Critical missing/unclear elements (as presented in the preprint text snippets available):
- Full strain/sex breakdown, housing conditions, diet, sentinel/pathogen status, and whether cohorts were contemporaneous.
- Randomization, blinding, and prespecified endpoints.
- Cause-of-death assessment, necropsy/histopathology, and cancer incidence—especially important given telomere manipulation claims.
- Survival analysis reporting beyond “Cox regression”: hazard ratios, confidence intervals, censoring rules, and whether investigators were blinded to treatment during monitoring.
Recommendation: Provide a CONSORT-style flow diagram for lifespan cohorts, complete metadata, necropsy summaries, and an independent replication (ideally at a second site).
2. Identity of the “telomere” cargo and risk of probe-driven or extracellular-DNA artifacts
The manuscript relies heavily on TelC PNA probes and flow cytometry/imaging of extracellular telomeric material.
Yet the authors also describe Rivers as residing in a large vesicle fraction and “likely due to NET-like structures,” which is a red flag for confounding by extracellular DNA/protein meshes, immune complexes, or cell death debris.
Recommendation: Add orthogonal validation that the nucleic acid is telomeric and particle-associated:
- Telomeric repeat qPCR with nuclease protection (DNase ± detergent) to distinguish surface-bound DNA vs encapsulated DNA.
- Southern blot / TRF-style assays, long-read sequencing, or hybridization assays on isolated material.
- EM/immunogold or super-resolution imaging showing telomeric DNA localization within bounded vesicles vs extracellular webs.
- Rigorous depletion controls for NETs (e.g., DNase treatment; neutrophil depletion models; NET markers such as citrullinated histones) and demonstration that “River” activity persists after NET disruption.
3. Mechanistic leap from circulating telomeric particles to multi-organ telomere elongation
The authors report measurable telomere elongation across organs and interpret this as rejuvenation.
But the mechanism by which exogenous telomeric DNA (from APCs) causes stable telomere lengthening in somatic tissues is not fully established within the evidence described. Alternative explanations include:
- Changes in immune tone/inflammation (IL-6 reductions) shifting cell population composition (e.g., fewer senescent immune infiltrates), which can affect bulk telomere readouts.
- Selection/expansion of longer-telomere subpopulations rather than true elongation per cell.
Recommendation: Provide:
- Single-cell or cell-type–resolved telomere measurements (e.g., Q-FISH on defined parenchymal cell types).
- Uptake/biodistribution data (which cells take up Rivers; receptor dependence).
- Tests for dependence on telomerase, DNA repair pathways, or end-joining/ALT-like mechanisms in recipient tissues.
4. “Stemness factor” interpretation and specificity
The proteomic enrichment for NOTCH/β-catenin/RUNX2/WNT5A is intriguing, but the interpretation as “stemness” cargo driving rejuvenation may be overextended without functional necessity tests in vivo.
The manuscript shows that silencing stem enzymes in T cells reduces their detection in Rivers (composition), but necessity for rejuvenation (function) is not clearly established in vivo from the excerpts.
Recommendation: Perform loss-of-function tests where Rivers are generated lacking specific candidate effectors (or recipients are blocked for NOTCH/WNT signaling) and demonstrate reduced rejuvenation/lifespan benefit.
5. Reproducibility and quantification of dosing
The paper often uses particle counts (e.g., ~5,000 particles/animal single dose) but the effective dose in terms of DNA amount, protein cargo, size distribution, and biological activity units remains underspecified.
Additionally, the text notes that in some experiments serum input volumes were not normalized “to preserve natural abundance differences,” but other places describe normalization steps (e.g., NTA-based normalization for artificial Rivers). These choices can complicate comparisons.
Recommendation: Standardize and report:
- Particle size distributions (NTA plots), DNA/protein mass per dose, and batch-to-batch variability.
- Clear dosing rationale and a dose–response curve for both rejuvenation markers and survival.
6. Incomplete disclosure around “DOS”
A “sMAC disruptor DOS” is used as a rejuvenating ex vivo CD4 intervention (10 µM, 4h), but the compound identity, selectivity, and off-target profile are not evident from the provided methods excerpt.
Given the centrality of this manipulation to the lifespan arm, this is a major transparency issue.
Recommendation: Provide full chemical identity, provenance, purity, stability, target engagement, and whether DOS has independent effects on recipients (even without Rivers).
Minor comments and clarifications
- Terminology: “Rivers” is memorable but anthropomorphic; consider a parallel formal term (e.g., telomeric EV networks) for clarity in archival literature.
- Cross-kingdom claims: The plant data are interesting but currently feel like a conceptual flourish; the manuscript should clarify what is truly homologous (biogenesis? composition? function?) vs merely analogous.
- Statistics reporting: Many analyses cite ANOVA + Bonferroni or t-tests; ensure assumptions, exact p-values, effect sizes, and whether tests were one- vs two-sided are reported uniformly.
- Controls for PKH67: Lipid dyes can label non-vesicular hydrophobic material; explicit dye-only controls and detergent sensitivity assays should be prominently shown.
Overall assessment and publication recommendation
Overall: This is an ambitious and potentially transformative preprint with a creative mechanistic proposal and a multi-layered experimental program.
But the central functional claims—especially multi-organ rejuvenation and dramatic lifespan extension—are sufficiently extraordinary that they currently require substantial additional validation, stronger orthogonal evidence of cargo identity, tighter exclusion of extracellular-DNA/NET confounds, and far more complete reporting for the survival cohorts.
Recommendation: Major revision (bordering on “resubmit as new”) prior to consideration in a high-impact venue. The manuscript could become a landmark, but only if the core claims withstand a more stringent evidentiary standard.
If you want, I can also produce (1) a point-by-point “reviewer #2”-style list of required experiments and (2) a short editor-facing summary stating the acceptability threshold for the lifespan claim.
claude:
Academic Review: “CD4⁺ T cells confer transplantable rejuvenation via Rivers of telomeres”
Overall Assessment
This preprint by Lanna and colleagues presents a provocative hypothesis that CD4⁺ T cells release extracellular telomeric structures called “Rivers” that systemically rejuvenate aged tissues and extend lifespan. While the concept is innovative and the experimental scope is extensive, the manuscript suffers from significant methodological concerns, unclear mechanisms, and claims that require substantial additional validation.
Major Strengths
1. Novel Conceptual Framework
The identification of extracellular telomeric structures (“Rivers”) as potential mediators of systemic rejuvenation represents a genuinely original contribution to aging biology. The cross-kingdom conservation (mammals and plants) is intriguing.
2. Comprehensive Experimental Approach
- Multiple complementary techniques (flow cytometry, microscopy, proteomics, in vivo lifespan studies)
- Both human and mouse model systems
- Functional validation through adoptive transfer experiments
3. Metabolic Insights
The connection between fatty acid oxidation (FAO), ceramidase activity, and telomere transfer at immune synapses adds mechanistic depth to previous work from this group.
Major Concerns
1. Characterization and Identity of “Rivers”
Critical Issues:
- The term “Rivers” is poorly defined. Are these vesicle networks, NET-like structures, or something else? Figure 1b shows “vessel-like networks” but their biochemical nature remains unclear.
- The differential centrifugation scheme (3,000×g) captures a heterogeneous population. Rivers are described as both “large vesicles” and “NET-like structures” (ED Fig. 2), which is contradictory.
- Flow cytometry gating on PKH67⁺TelC⁺GAPDH⁻ particles doesn’t definitively establish these are distinct entities from exosomes or other extracellular vesicles.
Recommendation: Comprehensive biophysical characterization (electron microscopy, nanoparticle tracking analysis, cryo-EM) is essential to establish Rivers as a distinct class of extracellular structure.
2. Telomere Measurement and Validation
Concerns:
- TelC probe specificity: While TZAP retention is mentioned (Supp Info 2, not shown), centromere controls only partially validate telomeric identity.
- Flow-FISH telomere measurements lack calibration standards in many experiments
- The claim that Rivers deliver functional telomeric DNA to recipient cells requires direct demonstration (e.g., integration assays, single-cell telomere tracking)
- How do extracellular telomeres escape degradation by serum nucleases?
3. Mechanistic Gaps
Unanswered Questions:
- How do Rivers reach target tissues? The paper shows systemic effects but provides no trafficking data.
- Cell-type specificity: Which cells in each organ take up Rivers? Flow cytometry (Fig 4c) suggests broad uptake but lacks resolution.
- Why is GAPDH exclusion critical? The proteomic data show GAPDH depletion, but the functional significance beyond “competitive loading” is unclear.
- Stemness factor transfer: The enrichment of NOTCH1, β-catenin, and RUNX2 is shown, but whether these proteins are functionally delivered and active in recipient cells is not demonstrated.
4. Asymmetric Division Claims
The evidence for AD-like behavior (Fig 3c-d) is circumstantial:
- CPT1A asymmetry in fixed cells doesn’t prove functional asymmetric segregation
- The connection between AD and River production relies heavily on anti-LFA1 blockade, which has pleiotropic effects on T cell function
- No live-cell imaging to track division outcomes
5. Lifespan Extension Data (Figure 5b)
Major Statistical and Design Issues:
- Sample sizes: N=10 per group for lifespan extension is modest. The “transplant-free old” control (n=26) is larger, creating statistical imbalance.
-
Median lifespan extension: ~17 months from a 20-month baseline is impressive, but:
- No survival table or hazard ratios provided
- “Several mice living to ~58 months” is anecdotal without individual survival data
- The DOS (drug) control shows similar effects to Rivers, raising questions about specificity
- Single intervention: Rivers were administered once. Long-term stability and therapeutic durability are unclear.
- Husbandry details: Were mice housed under specific pathogen-free conditions? Barrier housing? These profoundly affect lifespan.
6. Proteomics Analysis
DIA (Fig 3a):
- Only n=3 donors per group is underpowered for proteomic discovery
- Heatmap shows protein enrichment/depletion but lacks statistical testing (FDR, p-values)
- The “stemness pathway” enrichment (Supp Info 4, not shown) needs Gene Ontology or GSEA validation with statistical thresholds
DDA (Fig 3b):
- N=8 donors is better, but the TelC-biotin pulldown may co-precipitate non-telomeric DNA-binding proteins
- Absence of negative control (e.g., non-telomeric probe) is a critical omission
7. Artificial Rivers
The siGAPDH approach (Fig 4d-f) is clever but raises concerns:
- If GAPDH silencing alone creates “River-like” particles, are natural Rivers merely GAPDH-depleted APC vesicles?
- This undermines the specificity of the T cell-mediated River generation pathway
- The phenotypic effects (Fig 4f) are subjective without blinded assessment
8. Plant Data
The fenofibrate/xylem results (Fig 1e) feel tangential:
- Orchid xylem as a model for mammalian immune rejuvenation is a significant conceptual leap
- The connection between photosynthesis-driven β-oxidation and immune telomere transfer is not mechanistically justified
- This section could be removed without weakening the core mammalian story
Minor Concerns
9. Reproducibility and Transparency
- Many critical datasets are in “Supplementary Information” files (1-7) that are referenced but not provided for review
- Methods lack detail in places (e.g., “FAVS sorting” parameters, antibody clones/dilutions incomplete)
- No data availability statement for proteomics raw files
10. Senescence Markers
- β-galactosidase (SA-β-Gal) is a controversial senescence marker with high false-positive rates
- p16 and IL-6 are more reliable, but their reduction doesn’t prove reversal of senescence vs. clearance of senescent cells
- sMAC (sestrin2⁺p-p38⁺) is from the authors’ prior work and lacks independent validation
11. Statistical Analysis
- Extensive use of one-way ANOVA with Bonferroni correction is appropriate, but some comparisons lack sufficient rationale
- Missing statistical parameters (F-values, degrees of freedom) in figure legends
- No power calculations provided
12. Writing and Presentation
- The manuscript is dense and would benefit from streamlining
- Figures are information-rich but sometimes overwhelming (e.g., Extended Data Fig 10 has 4 panels with 5 conditions each)
- The “Rivers” metaphor, while evocative, may be more poetic than scientifically precise
Specific Technical Questions
- Figure 2c: How was “antigen-specific telomere release” quantified on bilayers? Background subtraction from peptide-free APCs is mentioned, but raw counts would clarify magnitude.
- Figure 3e: The “loss of supernumerary telomeres” is calculated as ΔTelC fluorescence (48-72h). Why this time window? Could this reflect probe dissociation rather than active disposal?
- Figure 4b: The CD4-depletion validation (91.1% to 0%) is excellent, but what about residual CD4⁺ cells in lymphoid tissues?
- Figure 5a: CPT1A overexpression restores River production in old T cells. Is this due to FAO restoration or CPT1A-independent effects (e.g., proliferation)?
- Methods - River transplants: “~5,000 particles” per mouse from 140 μL serum. How was this quantified? Nanoparticle tracking? Flow cytometry beads?
Missing Experiments
To strengthen the manuscript, the following are essential:
- Direct demonstration of telomere transfer: Use orthogonal DNA labeling (e.g., EdU incorporation in donor APC telomeres) to track functional integration into recipient cells.
- Mechanism of uptake: Which endocytic pathways mediate River internalization? Receptor identification?
- Dose-response: Does River transplant efficacy scale with particle number?
- Repeated dosing: Can multiple River administrations extend lifespan further?
- Telomerase-deficient models: Test Rivers in TERT⁻/⁻ mice to confirm telomerase-independent effects.
- Blinded histology: Fur quality and “rejuvenated appearance” (Fig 4f) require blinded scoring by independent observers.
- Human relevance: Are Rivers detectable in young vs. aged human serum at different levels?
Ethical and Conflict of Interest Considerations
- The senior author (A.L.) is CEO and shareholder of Sentcell Ltd, which holds patents on DOS and artificial River generation methods. This represents a significant COI.
- The lifespan extension claims have obvious translational implications. Independent replication by another lab is critical before clinical consideration.
Verdict
Recommendation: Major Revision
This manuscript presents an ambitious and potentially paradigm-shifting model of immune-mediated systemic rejuvenation. However, the evidence is currently insufficient to support the strong claims made, particularly regarding lifespan extension and the mechanistic basis of River function.
Key Requirements for Resubmission:
- Rigorous biophysical characterization of Rivers
- Direct demonstration of functional telomere transfer
- Mechanistic data on River trafficking and cell-type specificity
- Independent replication of lifespan extension with larger cohorts
- Complete transparency with supplementary data and raw files
Suitability:
If these concerns are addressed, this work could represent a major advance in aging biology, suitable for a high-impact journal (Nature, Cell, Science). In its current form, it requires substantial additional validation.
Significance if Claims are Validated
If the authors’ model is correct—that CD4⁺ T cells distribute telomeric and stemness cargo systemically to rejuvenate tissues—this would represent:
- A new paradigm for immune system regulation of organismal aging
- A potential therapeutic avenue (artificial Rivers) for age-related diseases
- A mechanistic explanation for parabiosis effects
However, extraordinary claims require extraordinary evidence, and this manuscript, while provocative, has not yet reached that threshold.
Reviewed by: [Anonymous Academic Reviewer]
Date: January 23, 2026
Expertise: Immunology, Aging Biology, T Cell Biology
gemini:
Formal Academic Review
Title: $CD4^{+}$ T cells confer transplantable rejuvenation via Rivers of telomeres
Authors: Alessio Lanna, Salvatore Valvo, Michael L. Dustin, Federica Rinaldi
Date of Review: January 23, 2026
Source Material: bioRxiv preprint 2025.11.14.688504
1. Executive Summary
This study by Lanna et al. proposes a novel, systemic rejuvenation mechanism mediated by the immune system. The authors demonstrate that following antigen-specific activation, $CD4^{+}$ T cells release distinct extracellular vesicles containing telomeric DNA—termed “telomere Rivers”—into the circulation. The central hypothesis is that these vesicles act as a “transplantable program of youth,” transferring telomeres and stemness factors (such as NOTCH1 and WNT5A) to non-immune tissues (brain, liver, heart), thereby reversing senescence and extending lifespan.
Building on the authors’ previous work regarding telomere transfer from antigen-presenting cells (APCs) to T cells, this paper argues that T cells act as a vector to redistribute these telomeres systemically. The study identifies a metabolic checkpoint involving Fatty Acid Oxidation (FAO) and the exclusion of the enzyme GAPDH as critical for the formation of functional, rejuvenating Rivers. The authors claim an unprecedented median lifespan extension of approximately 17 months in aged mice treated with these vesicles.
2. Assessment of Significance and Novelty
Novelty: The manuscript presents a highly original paradigm. While parabiosis studies have long suggested circulating factors control ageing, identifying specific, immune-derived particulate carriers (telomeric vesicles) that cross organ boundaries is a significant departure from canonical soluble factor theories (e.g., SASP dilution). The concept of T cells serving not just as immune effectors but as “youth-promoting” signal transducers adds a new dimension to immunometabolism and gerontology.
Significance: If validated, the findings are transformative. The ability to generate “Artificial Rivers” by silencing GAPDH in APCs suggests a scalable, cell-free therapeutic avenue for age-related multimorbidity. The observation of conserved mechanisms in plants hints at a fundamental biological signaling pathway, though this breadth also invites skepticism regarding the specificity of the mechanism.
3. Critical Evaluation of Methodology
Strengths:
- Multi-modal Approach: The study utilizes a robust combination of high-resolution microscopy (IF-FISH), advanced proteomics (DIA and DDA), flow-FISH, and in vivo longevity studies.
- Mechanistic Dissection: The authors go beyond phenomenology to identify the molecular machinery required for vesicle formation, pinpointing the role of CPT1A-driven FAO, the ceramide-PE axis, and the specific exclusion of GAPDH.
- “Artificial” Controls: The creation of synthetic Rivers via GAPDH silencing in APCs serves as a powerful gain-of-function control, isolating the vesicle composition as the causative agent of rejuvenation.
Weaknesses and Areas for Scrutiny:
- Nomenclature and Definition: The term “Rivers” is metaphorical and non-standard. While the authors define them as “elongated, punctate structures… in vessel-like networks” and later as vesicles in the 3,000 xg pellet, the physical characterization (size distribution, markers beyond TelC/PKH67) requires strict adherence to ISEV (International Society for Extracellular Vesicles) guidelines to distinguish them from apoptotic bodies or large oncosomes.
- Tissue Uptake Mechanism: The paper demonstrates that Rivers rejuvenate distant organs (brain, liver), but it lacks a detailed mechanism for how these large telomeric complexes cross endothelial barriers (particularly the blood-brain barrier) and are internalized and integrated by parenchymal cells.
- Cross-Kingdom Claims: The inclusion of plant (orchid) data claiming similar “River” structures in xylem sap is provocative but under-characterized compared to the mammalian data. It risks diluting the focus of the immunological mechanism.
4. Analysis of Results and Data Interpretation
A. The Metabolic Checkpoint (FAO and GAPDH): The authors provide convincing data that senescent T cells ($T_{sen}$) fail to produce Rivers due to a metabolic block in FAO. Restoring CPT1A expression or supplementing phosphatidylethanolamine (PE) rescues synapse formation and telomere acquisition. The finding that functional Rivers are GAPDH-low while non-functional vesicles are GAPDH-high is a crucial distinction. The proteomics data supporting the enrichment of stemness factors (Runx2, Notch, Wnt) in Rivers is compelling.
B. Asymmetric Division-like Behavior: The data suggests that T cells process APC-derived telomeres via an asymmetric division-like mechanism, segregating CPT1A and telomeres into specific daughter vesicles. This provides a logical intracellular mechanism for how “spare” telomeres are packaged for export.
C. In Vivo Rejuvenation and Lifespan: The lifespan extension data is extraordinary. Treatment extended median lifespan by ~17 months, with maximum survival reaching ~58 months. In typical C57BL/6 colonies, median survival is ~26-30 months. A 17-month extension represents a roughly 60% increase, which supersedes effects seen in rapamycin or caloric restriction studies. Such magnitude requires rigorous independent replication. The reduction in senescence markers (p16, $\beta$-gal) across multiple organs supports the survival data.
D. Artificial Rivers: The experiment showing that GAPDH-depleted APC vesicles (“Artificial Rivers”) recapitulate the rejuvenation phenotype rules out the absolute requirement for T-cell processing if the vesicle composition is artificially engineered. This creates a strong case for the “cargo” (telomeres + stem factors - GAPDH) being the functional unit.
5. Ethical and Translational Considerations
- Safety: The introduction of large amounts of telomeric DNA and stemness factors (Wnt/Notch) poses a theoretical cancer risk, although the authors note the animals lived longer without reporting increased tumor incidence. The disposal of “supernumerary” telomeres suggests a homeostatic mechanism, but the long-term genomic stability of recipient tissues needs assessment.
- Translation: The study utilizes human T cells and plasma, suggesting immediate translational relevance. The “Artificial River” approach allows for a pharmaceutical strategy that bypasses the need for autologous T-cell manipulation.
6. Conclusion and Verdict
Verdict: Major Significance / High Impact Potential
This manuscript presents a paradigm-shifting discovery linking adaptive immunity, metabolism, and systemic ageing. The identification of “Rivers” as specific, metabolically regulated, telomere-carrying vesicles offers a concrete mechanism for the “young blood” effects observed in parabiosis.
Recommendations for Revision:
- Characterization: Provide electron microscopy or high-resolution imaging of the “River” particles to better define their structure compared to canonical extracellular vesicles.
- Uptake Mechanism: Investigate how these vesicles enter non-phagocytic cells (e.g., neurons or cardiomyocytes) and how the telomeric DNA acts within the recipient nucleus (integration vs. transient signaling).
- Plant Data: Consider moving the plant data to a supplementary section or a separate brief communication to maintain focus on the mammalian immune mechanism, unless the evolutionary conservation is mechanistically detailed.
- Statistics: Ensure the survival analysis (Cox regression) accounts for all covariates, given the massive effect size reported.
Final Note: If reproducible, the “River” therapy described here represents one of the most potent anti-ageing interventions reported to date.
