That’s a nice hypothesis but that’s not evidence. True scientists always question outdated axioms. Your whole argument is anchored on an assumption that we know what the is the human limit, but until you can perform the actual experiment (which will never happen obviously) you really don’t know, do you? You just cling to this unproven idea and rationalize it any way you can.
Humans may not have the traditional animal predators that mice have, but we are exposed daily to pollutants, toxins, irritants, infectious agents, stress and other non-traditional “predators”. How do you know if the humans were in a controlled laboratory settings FREE of all those agents, if they would not achieve a much higher life span?
Actually microbial infections can induce many of those states. Here is just one example. I don’t want go on forever.
Possible Pathogenesis and Prevention of Long COVID: SARS-CoV-2-Induced Mitochondrial Disorder
Tsung-Hsien Chen,1 Chia-Jung Chang,2,* and Peir-Haur Hung1,3,*
João R. Mesquita, Academic Editor
Author information Article notes Copyright and License information PMC Disclaimer
Associated Data
Abstract
Patients who have recovered from coronavirus disease 2019 (COVID-19) infection may experience chronic fatigue when exercising, despite no obvious heart or lung abnormalities. The present lack of effective treatments makes managing long COVID a major challenge. One of the underlying mechanisms of long COVID may be mitochondrial dysfunction. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections can alter the mitochondria responsible for energy production in cells. This alteration leads to mitochondrial dysfunction which, in turn, increases oxidative stress. Ultimately, this results in a loss of mitochondrial integrity and cell death. Moreover, viral proteins can bind to mitochondrial complexes, disrupting mitochondrial function and causing the immune cells to over-react. This over-reaction leads to inflammation and potentially long COVID symptoms. It is important to note that the roles of mitochondrial damage and inflammatory responses caused by SARS-CoV-2 in the development of long COVID are still being elucidated. Targeting mitochondrial function may provide promising new clinical approaches for long-COVID patients; however, further studies are needed to evaluate the safety and efficacy of such approaches.
The number of centenarians in Hong Kong has almost doubled in the past two decades. That’s not genes. They rank 4th globally. Japan is the king at 76.5 centenarians per 100,000 residents. India is the worst at 2.1 centenarians per 100,000 residents. The USA has 25 centenarians per 100,000 residents.
It found that Hong Kong, whose centenarian population rose by about 90 percent in those two decades, was home to an estimated 3,561 centenarians in 2020 or 47 super seniors for every 100,000 residents.
After comparing assessments with other countries, the researchers found that Hong Kong’s longevity is attributed to its low mortality rate for cardiovascular diseases in men and women.
HK's hearty longevity edge | The Standard.
The United Nations estimated that there were 316,600 living centenarians worldwide in 2012, and 573,000 in 2020, almost quadruple the 2000 estimate of 151,000.
It seems lifespans have not increased since ancient times until the post-WWII era if you exclude child and infant mortality.
A survey of the lifespans of male individuals with entries in the Oxford Classical Dictionary (i.e., a sample pre-selected to include those who lived long enough to attain historical notability) found a median lifespan of 72 years, and a range of 32 to 107 years, for 128 individuals born before 100 BC (though the same study found a median lifespan of 66 years for 100 individuals born after 100 BC but no later than 602 AD); by comparison, male individuals listed in Chambers Biographical Dictionary who died between 1900 and 1949 had a median lifespan of 71.5 years, with a range between 29 and 105 years.
I find the number per 100k confusing and even irrelevant since that doesn’t takes into account the average age for that nation. As an example, if the national average age for a country is 30 of course there wouldn’t be many centenarians (as a % or out of 100k) in that country since MOST of their people were born in last 50 years. Whereas a country with an average age of 60 there’s got to be way more centenarians as a percentage of population.
A more precise number would be the percentage of people that make it to 100, let’s say how many people were born in 1924, and how many of them (as a percentage, or out of 100000) are alive today. Any other measure is somewhat misleading.
More evidence of pathogens shortening our healthspans.
Scientists Trace Lupus to One of The World’s Most Common Viruses
Health14 November 2025
 (Kateryna Kon/Sciepro/Science Photo Library/Getty Images)
(Kateryna Kon/Sciepro/Science Photo Library/Getty Images)
One of the world’s most common viral infections could underlie virtually every case of lupus, according to a recent study providing the strongest evidence yet for a link.
The research, led by scientists at Stanford University, has found that the Epstein-Barr virus (EBV) could be the trigger behind the ‘cruel mystery’.
EBV is the pathogen that causes ‘kissing disease’ (or mononucleosis), and according to the new findings, it can directly infect and reprogram specific immune cells, potentially driving the onset of the chronic autoimmune disease systemic lupus erythematosus – better known as simply lupus.
“This is the single most impactful finding to emerge from my lab in my entire career,” claims immunologist and head of the lab William Robinson.
“We think it applies to 100 percent of lupus cases.”
Related: Serotonin Could Play an Unexpected Role in Cancer, Scientists Discover
The vast majority of adults in the world have been exposed to EBV at some point in their lives, with the virus causing little to no fuss as it lies latent in their body’s cells.
Those with lupus, however, show a deeper infection, possibly because they acquired a more virulent EBV strain. Among patients with the autoimmune condition, researchers found the percentage of B cells infected with EBV is about 1 in 400. That’s 25 times higher than in healthy individuals.
In the lab, the infection flicked a switch in B cells, activating a system that ‘turns on’ their pro-inflammatory genes.
This has the potential, “to promote systemic disease–driving autoimmune responses,” the authors argue, led by immunologist Shady Younis from Stanford.
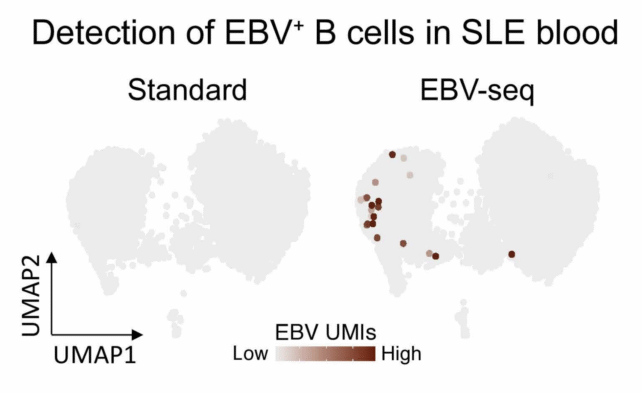
EBV sequencing enabled identification of EBV-infected B cells in the blood of those with and without lupus. (Younis, Sci. Trans. Med., 2025)
The discovery could help solve the long-standing mystery of what triggers lupus in the first place and why its symptoms go through seemingly random cycles of flaring and settling.
Lupus causes the immune system to mistakenly attack the body’s own healthy tissues, causing widespread inflammation throughout the body with potentially severe, life-threatening outcomes.
The disease was first mentioned in historical records way back in 850 CE, and yet to this day, there is no known cause or cure. Only in the 19th century did experts officially recognize and describe lupus, which can cause a rash resembling a wolf’s bite (hence the historical Latin name).
The enduring mystery of lupus is often pinned down to its complex nature, possibly triggered by numerous interacting factors, such as nutrient deficiencies, genetics, hormonal issues, or infections.
The new research from Stanford suggests there really may be a unifying explanation – one with viral origins.
Related: Lupus Tracked to Changes in a Single Gene That Tames The Immune System
For years now, researchers have suspected that EBV is linked to lupus. The virus is known to infect B cells, and B-cell activity is known to be imbalanced in those with lupus. The trouble is, EBV is hard to measure when it infects and hides out in B cells.
Researchers at Stanford devised a clever way to find which of these white blood cells are infected with the virus.
Using their new sequencing technique, the team demonstrated that people with lupus have significantly more EBV-infected B cells than those without lupus, especially memory B cells, which enable rapid immune responses.

Memory B cells ‘remember’ specific pathogens so they can launch quick immune responses. (Synscicomics)
Of all the hundreds of billions of B cells in a healthy human body, only about 20 percent are ‘autoreactive’, or primed to produce antibodies and activate killer immune cells.’ But when EBV infects latent B cells, it appears to flip them back into a pro-inflammatory state.
“Our findings provide a mechanistic basis for why only a small fraction of EBV-infected individuals develop SLE,” the authors conclude.
The mechanism is supported by a recent immunotherapy for lupus, which hunts down and replaces faulty B cells. It showed remarkable benefits in clinical trials, achieving remission-like outcomes.
Virologist Guy Gorochov from Sorbonne University in France, who was not involved in the current study, told The Guardian’s Hannah Devlin that the work was "impressive.
“It’s not the final paper about lupus,” he added, “but they’ve done a lot and developed an interesting concept.”
Going forward, the findings may even be relevant to other autoimmune conditions linked to EBV, such as multiple sclerosis, long COVID, and myalgic encephalomyelitis/chronic fatigue syndrome.
“Practically the only way to not get EBV is to live in a bubble,” Robinson says.
The study was published in Science Translational Medicine.