We should test the non viral vector using hTERT therapy in mice and move this into human…
Another good article by Bill Haseltine:
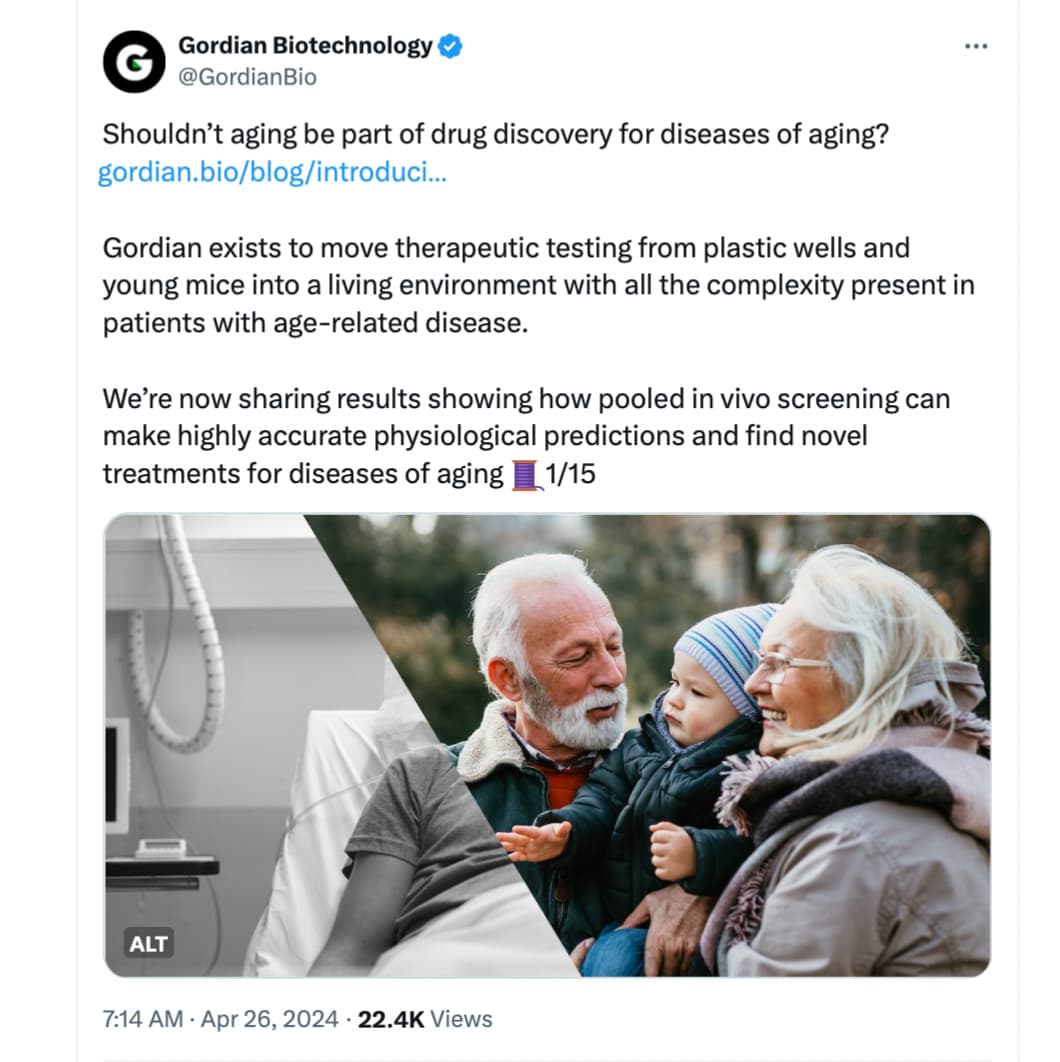
Longevity biotech company Gordian Biotechnology launches their in vivo screening platform…
Forbes magazine coverage:
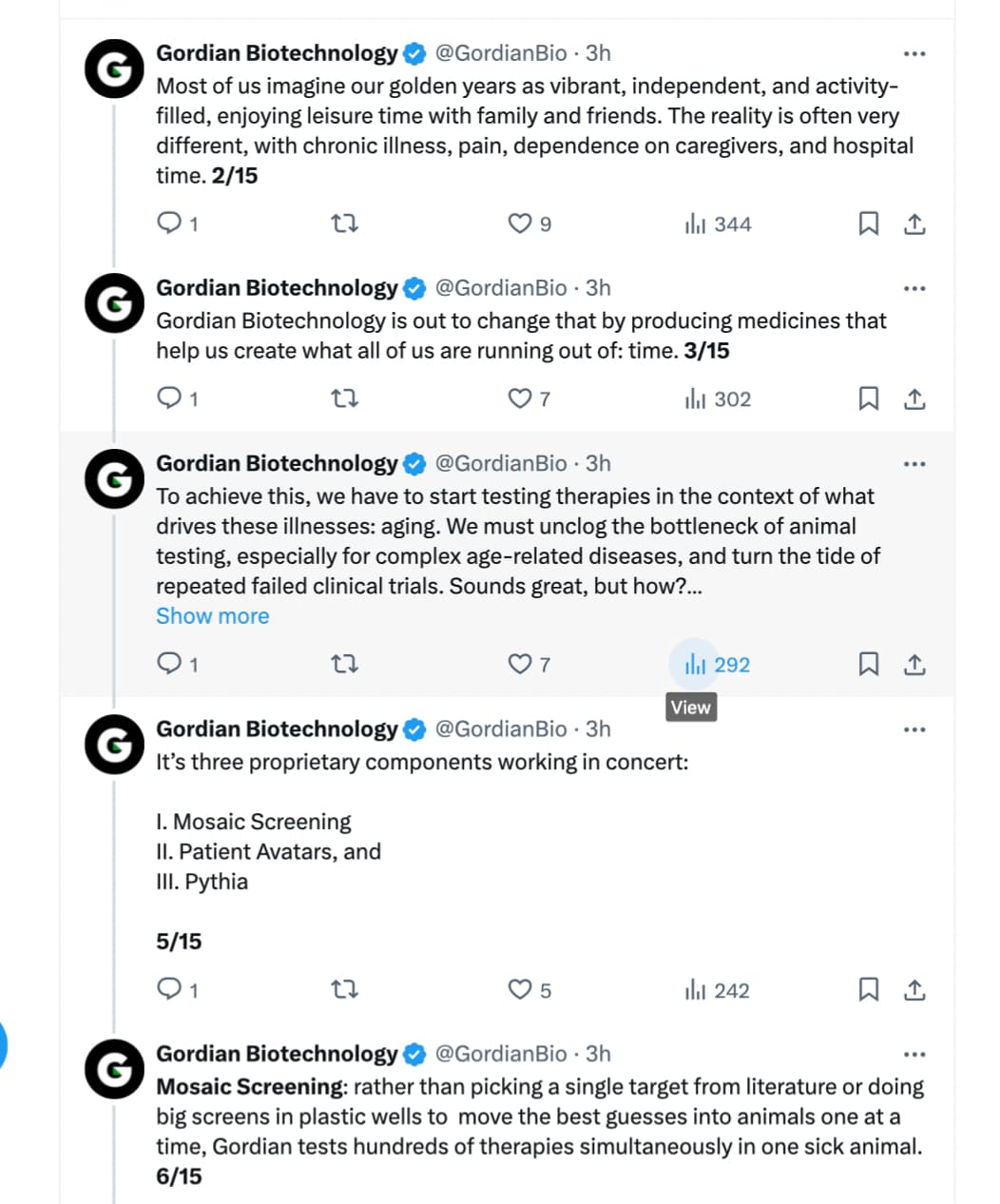
When developing new therapies for diseases, biotech researchers are often limited by two time-consuming steps: first, screening thousands of drug candidates in test tubes and second, taking the best candidates and testing them on multiple animals to make sure it’s safe and effective. Combined, these steps can be slow and expensive.
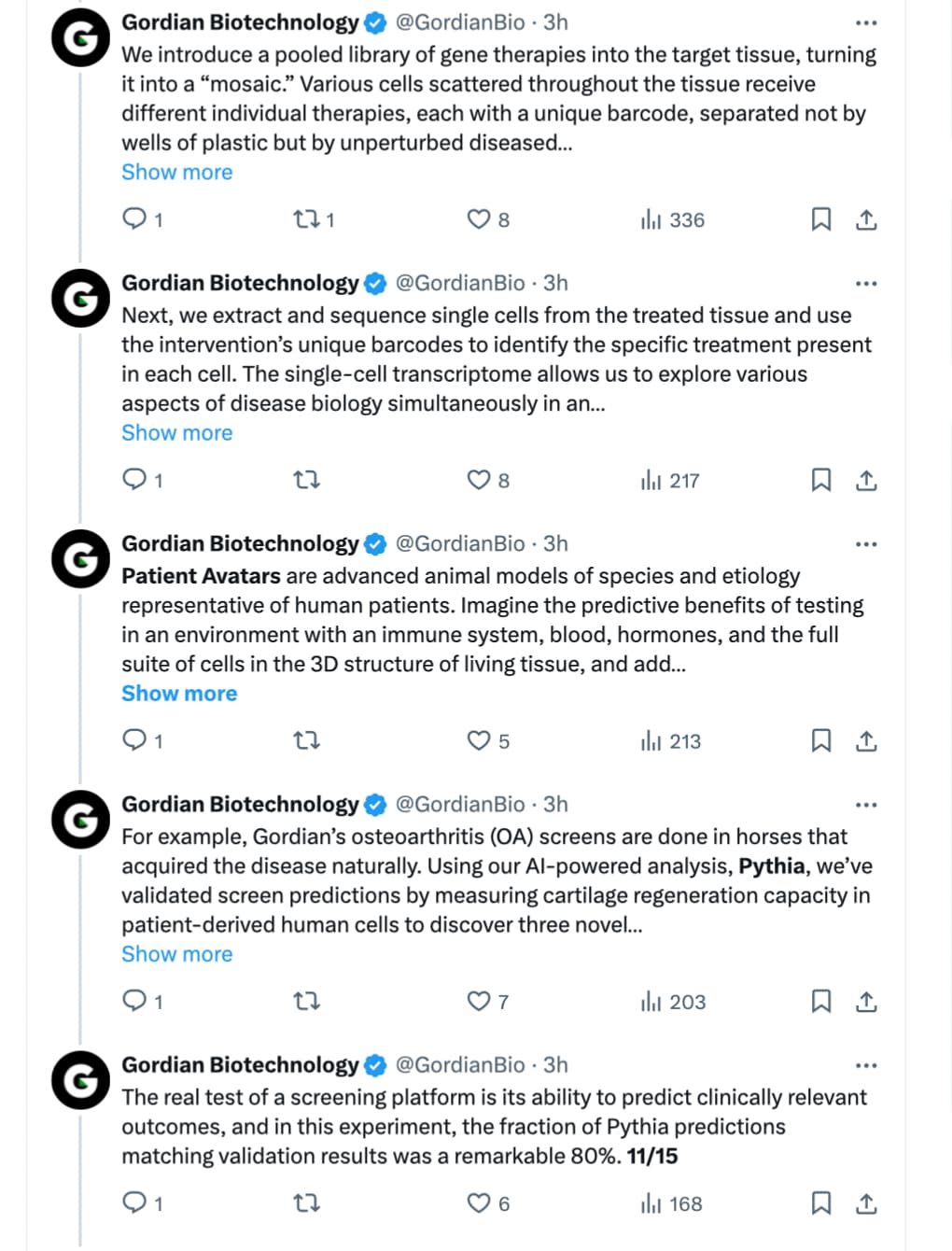
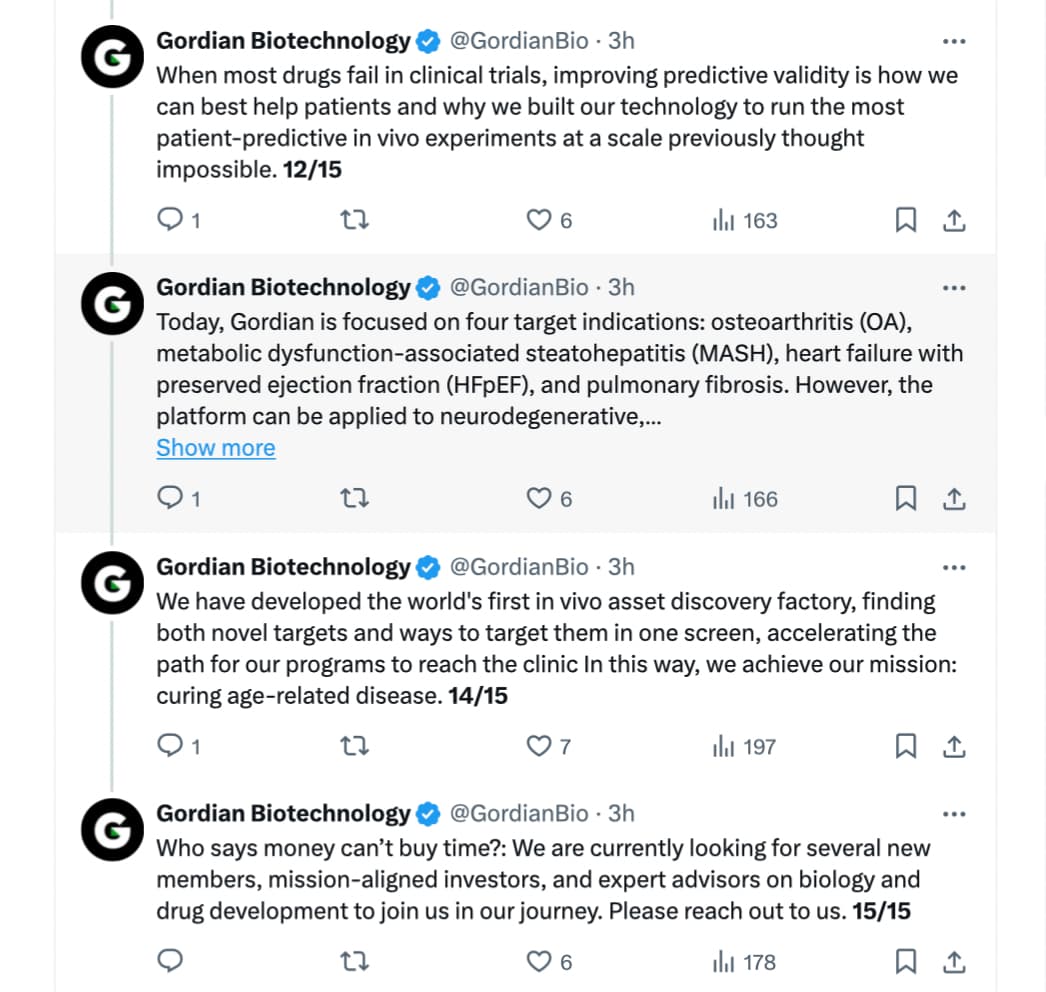
Today, a startup called Gordian Biotechnology debuted a technology that could make this process better for both animals and people. The San Francisco-based company has developed a new animal screening platform which allows multiple gene therapies to be tested at the same time with just one animal. Instead of the gene treatment being given to the animal and affecting an entire area of its body, Gordian’s innovation enables it to test a drug inside of a single cell. That means one mouse could potentially support the evaluation of hundreds of possible new gene therapies in a way that’s faster and impacts fewer animals with less risk of harm to them.
Full story: This Biotech Startup Aims To Speed Up Drug Testing On Animals
The company website information on their platform:
More details in this Twitter / X thread:
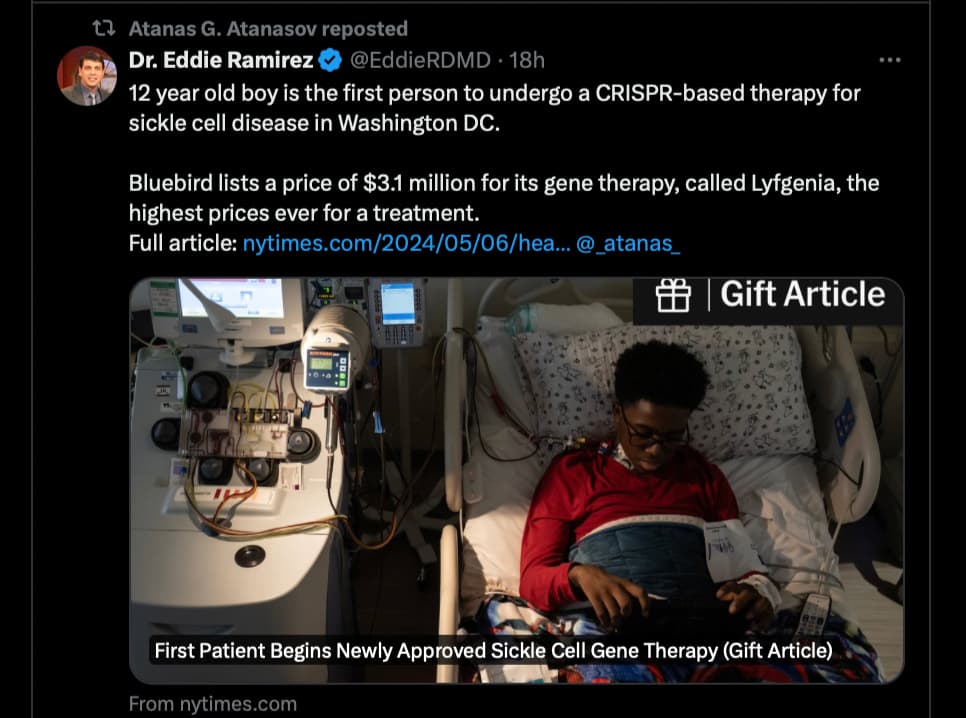
I assume that the boy gets the treatment for free as a “human guinea pig” discount.
Seems like a treatment only the top 0.1% can afford.
Gordian Biotechnology is introducing a method of screening hundreds of gene therapies simultaneously in an individual animal model - its co-founders explain
“Every time we approve a new gene therapy, and then the company announces the price of the gene therapy, the commissioner sends me a note and it’s usually [short]—I love short emails,” Marks told the crowd at the American Society of Gene and Cell Therapy annual meeting Wednesday morning. Marks serves as director of the Center for Biologics Evaluation and Research (CBER).
“The last one was a $4.25 million exclamation point," Marks recalled. "It was like a lot of exclamation points after. I can’t remember how many, and that was the email. And I knew what it meant.”
CBER does not consider prices in approval decisions for the therapies it reviews, Marks said. It’s just not part of the group’s remit.
“On the other hand, it’s my job to look at the entire ecosystem," he commented Wednesday. "Step back independent of a given product and say, is this sustainable? In other words, can we ultimately have the field of gene therapy mature and start to treat more common diseases if we keep having to charge a million or $2 million for a dose?”
Every single technology starts expensive and gets cheaper to benefit all.
If you ban expensive technology you don’t get cheaper versions - you get no technology at all. Price caps lead to empty shelves, not cheap goods.
The only way we can “ultimately have the field of gene therapy mature and start to treat more common diseases” is by letting them charge as much as they want like all other free market products.
Not to mention - the only regulation that should exist is for safety, fraud prevention - and even that is debatable - how many people’s lives will be shorter with more disease because you need an authority approval to buy rapamycin?
Really interesting. Making progress.
Gene Therapy For Blood Diseases
What if we could repair fundamental parts of our bodies?Gene therapy promises just that. It’s like having a molecular mechanic that can fix or replace faulty genes, potentially curing diseases previously thought to be incurable. One area where gene therapy is making significant progress is in treating blood disorders. It offers hope to people with sickle cell disease or hemophilia.
More recently, we have even seen success in implementing therapies directly in the body, a process known as in vivogene editing. It’s like performing microsurgery on your DNA while it’s still inside you. This approach could open doors to treating many diseases with a single procedure. One study recognized and explored the potential of this in vivo therapy for blood disorders and diseases. The research paper published in Science discusses a groundbreaking approach to altering hematopoietic stem cells within the body.
This is a huge step forward. The trick is going to be finding a way to speed up the safety and regulation bottleneck.
Good news on the gene therapy front:
Source: x.com
Full Paper:
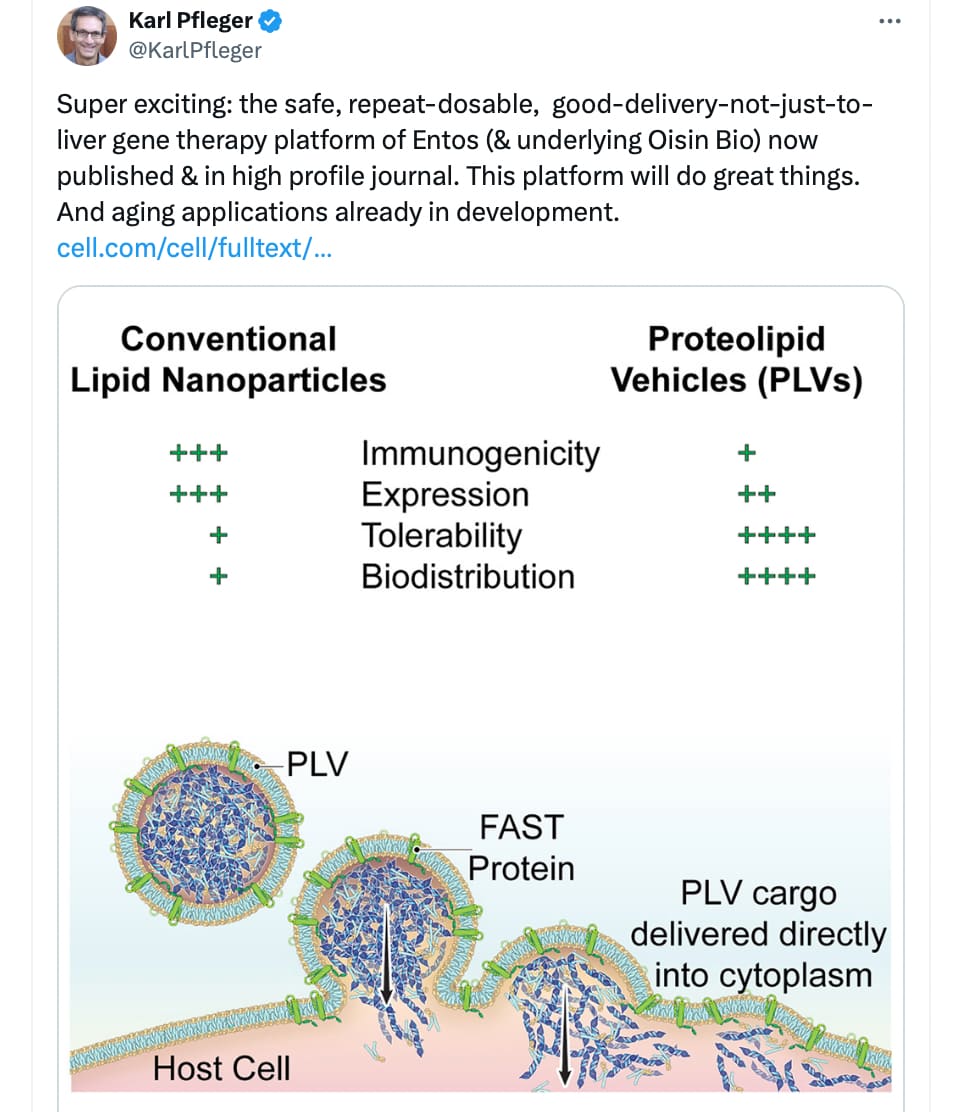
Safe and effective in vivo delivery of DNA and RNA using proteolipid vehicles
I’m also bullish on FusoGenix. I invested in Aegis (investment in Entos was not available or I would have done that)
Probably this link is relevant for the subject:
Baby Is Healed With World’s First Personalized Gene-Editing Treatment
Something was very wrong with Kyle and Nicole Muldoon’s baby.
The doctors speculated. Maybe it was meningitis? Maybe sepsis?
They got an answer when KJ was only a week old. He had a rare genetic disorder, CPS1 deficiency, that affects just one in 1.3 million babies. If he survived, he would have severe mental and developmental delays and would eventually need a liver transplant. But half of all babies with the disorder die in the first week of life.
Instead, KJ has made medical history. The baby, now 9 ½ months old, became the first patient of any age to have a custom gene-editing treatment, according to his doctors. He received an infusion made just for him and designed to fix his precise mutation.
The investigators who led the effort to save KJ are presenting their work on Thursday at the annual meeting of the American Society of Cell & Gene Therapy, and are also publishing it in the New England Journal of Medicine.
The implications of the treatment go far beyond treating KJ, said Dr. Peter Marks, who was the Food and Drug Administration official overseeing gene-therapy regulation until he recently resigned over disagreements with Robert F. Kennedy Jr., the secretary of health and human services. More than 30 million people in the United States have one of more than 7,000 rare genetic diseases. Most are so rare that no company is willing to spend years developing a gene therapy that so few people would need.
But KJ’s treatment — which built on decades of federally funded research — offers a new path for companies to develop personalized treatments without going through years of expensive development and testing.
Read the full story: https://www.nytimes.com/2025/05/15/health/gene-editing-personalized-rare-disorders.html?unlocked_article_code=1.HU8.bCz-.eYLm-hxkP5FG&smid=url-share
Details on how they did this quickly, a 15 minute interview with the key developer of the new therapy:
Breakneck breakthrough
The gene editing technology used for KJ is not exactly new, nor is the delivery system. CRISPR has already proven to be a successful gene therapy. What’s stunning is the speed. KJ’s mutation was identified within days of his birth. Within weeks, researchers were growing cells in petri dishes that carried genetic sequences copied from KJ. In month two, they used those cells to train molecular gene-editing machinery to target and correct KJ’s mutation—a spot in the DNA coding for the liver enzyme where there’s a T (thymine) instead of a C (cytosine). At the beginning of the third month, researchers had created genetically engineered mice that carry KJ’s specific mutation, too.
In the boy’s fourth month, researchers were meeting with the Food and Drug Administration to discuss regulatory approval for a clinical trial—a trial where KJ would be the only participant. They were also working with the institutional review board (IRB) at Children’s Hospital of Philadelphia to go over the clinical protocol, safety, and ethical aspects of the treatment. The researchers described the unprecedented speed of the oversight steps as being “through alternative procedures.”
In month five, they started toxicology testing in mice. In the mice, the experimental therapy corrected KJ’s mutation, replacing the errant A-T base pair with the correct G-C pair in the animals’ cells. The first dose provided a 42 percent whole-liver corrective rate in the animals. At the start of KJ’s sixth month, the researchers had results from safety testing in monkeys: Their customized base-editing therapy, delivered as mRNA via a lipid nanoparticle, did not produce any toxic effects in the monkeys.
A clinical-grade batch of the treatment was readied. In month seven, further testing of the treatment found acceptably low-levels of off-target genetic changes. The researchers submitted the FDA paperwork for approval of an “investigational new drug,” or IND, for KJ. The FDA approved it in a week. The researchers then started KJ on an immune-suppressing treatment to make sure his immune system wouldn’t react to the gene-editing therapy. Then, when KJ was still just 6 months old, he got a first low dose of his custom gene-editing therapy.
That is insane. We are almost living in a sci-fi future. If this keeps getting better and better, the possibilities are tremendous IMO. Hopefully they can also streamline the administrative and animal testing aspects to make the process even shorter - especially when KJ grows older and he takes part in studies to check the progression of the therapy. Then we’ll have a better understanding of the delivery efficiency etc.