I don’t know what the IP situation is when it comes to adding indications.
As far as I know, the same pleiotropic effects are observed with other GLP1-RAs in longitudinal studies and in clinical trials. Here’s an example of exenatide kidney benefits: Effects of exenatide and open-label SGLT2 inhibitor treatment, given in parallel or sequentially, on mortality and cardiovascular and renal outcomes in type 2 diabetes: insights from the EXSCEL trial 2019. For neuroprotection, exenatide, liraglutide, and lixisenatide were found to be beneficial in Parkinson’s disease (with a caveat for the phase 3 trial of exenatide: it seems that it was only beneficial in pre-diabetic people, which confirms my point that in perfectly healthy people these drugs might not be beneficial).
IP situation is very good and that’s why all companies rush to add indications as fast as possible. It’s free money. As you can see on the pipeline ( Experience with GLP-1s - #472 by adssx ) they ARE working hard to add indications. Including for CKD and HF. But ONLY combined with T2D and/or obesity. They must know something.
The once daily pills coming out next year will likely start new trials on them for Non-Diabetic/Obese individuals.
It’s as a easy as a vitamin and they can easily make low dose versions for these new indications.
Is the first anti-aging drug class on the horizon?
This is what Andrew Adams from Eli Lilly and Company had to say during this years hashtag#ARDD2025
Well, we know how the world works - people who are not overweight or diabetic will still use these drugs for other purposes, like trying to rebalance body composition, get rid of visceral fat, suppress appetite and so on, by the tens of thousands. This means that in time we’ll have a lot of clinical data on outcomes and longer term effects. Physicians will write up case reports and so on. Eventually we’ll find out what the effects are for non-diabetic normal weight people. Of course systematic controlled studies would be best, but pharma are not the only sources who can finance studies. There’s always academia and curious researchers who want to understand how things work. Who says we won’t see such studies down the line? There’s a lot of signals out there that these drugs are neuroprotective in certain contexts. I can’t believe nobody is interested in studying this in a targeted way.
See the above link. There is a huge interest in the potential for GLP1’s for longevity related concerns and not all peptides in the GLP1 family induce weight loss.
People are studying it: academia. And so far it seems that for Parkinson’s disease for instance the benefits are limited to pre-diabetic or diabetic people or people with some kind of insulin issues. Which is probably why none of the big pharma companies are funding trials in non T2D / non-obese people: these drugs might not work.
Interestingly, Novo Nordisk has an ongoing trial of NNC0519-0130, a dual GLP-1/GIP agonist, “in People With Chronic Kidney Disease, With or Without Type 2 Diabetes, and With Overweight or Obesity”. See NCT06717698. They’re comparing NNCx to semaglutide and placebo. Results at the end of 2026? However this recent article said the drug candidate has just been dropped: Novo Nordisk's 9,000-Job Cut and Strategic Restructuring: Assessing Long-Term Profitability and Competitive Positioning in the Obesity Drug Market ![]()
As the Popcorn Report predicted over 40 years ago, the aging population is going to drive the market for innovation.
Big Pharma leaders embrace GLP-1s as longevity drugs as debate grows on what this means for patients, pipelines and policy.
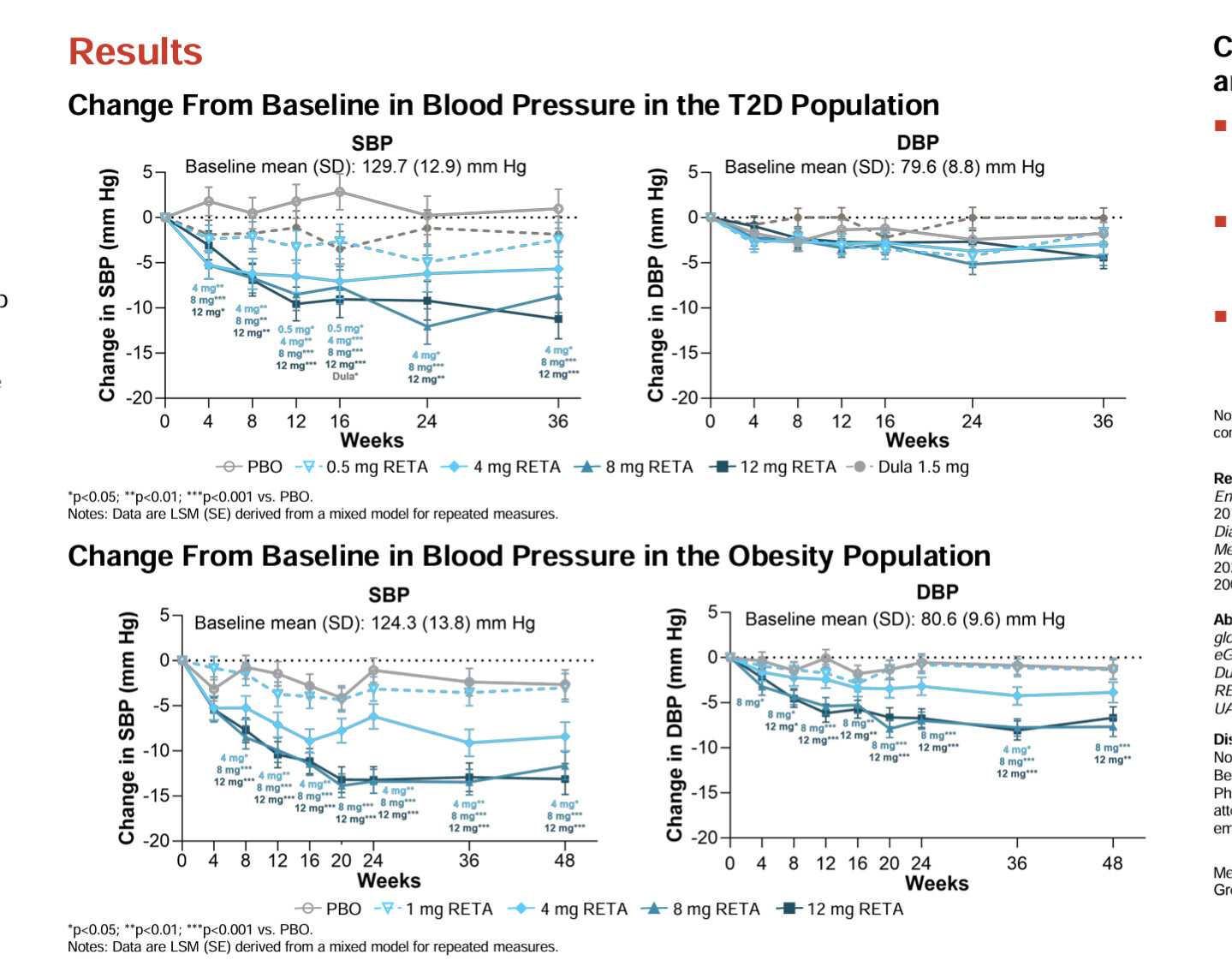
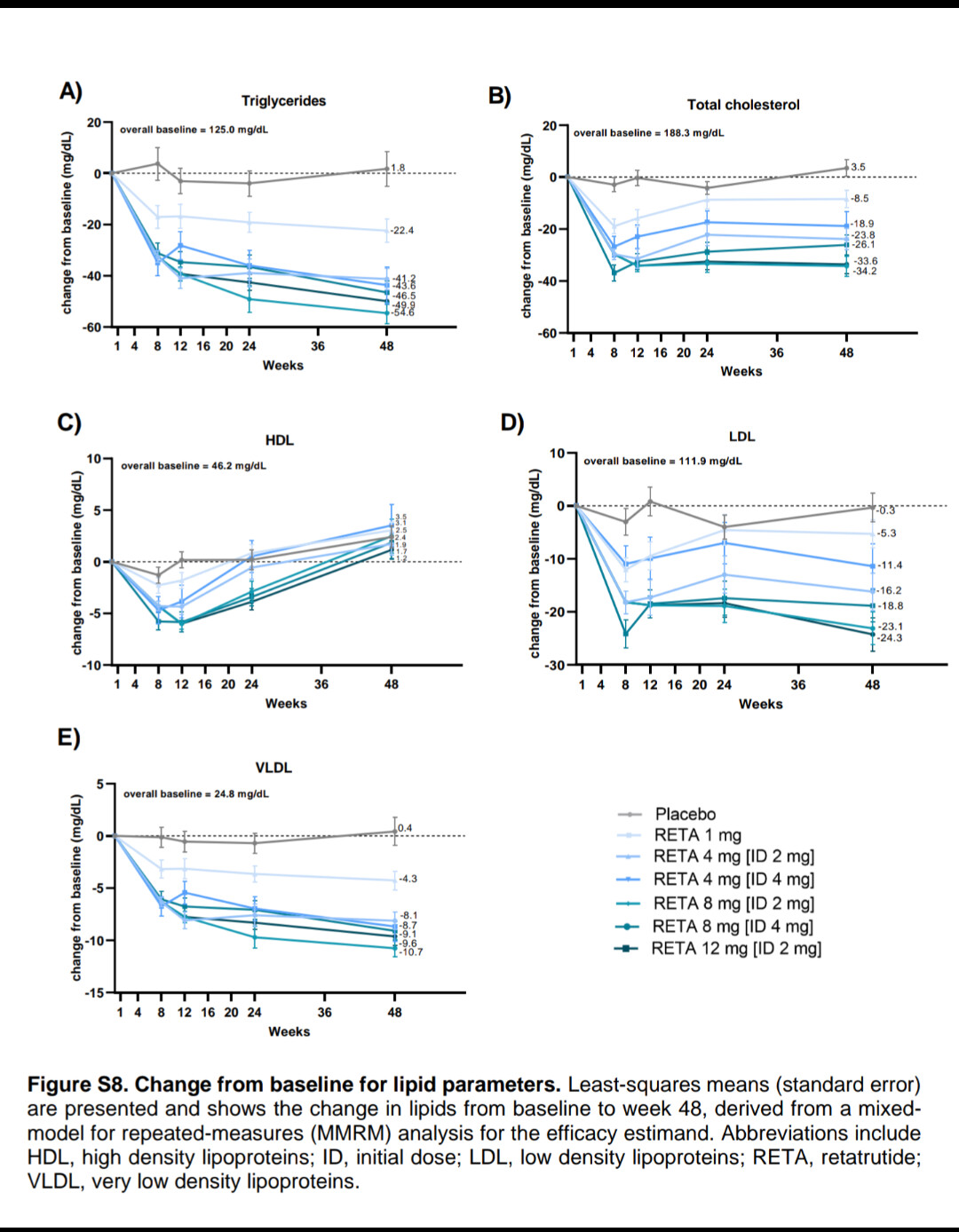
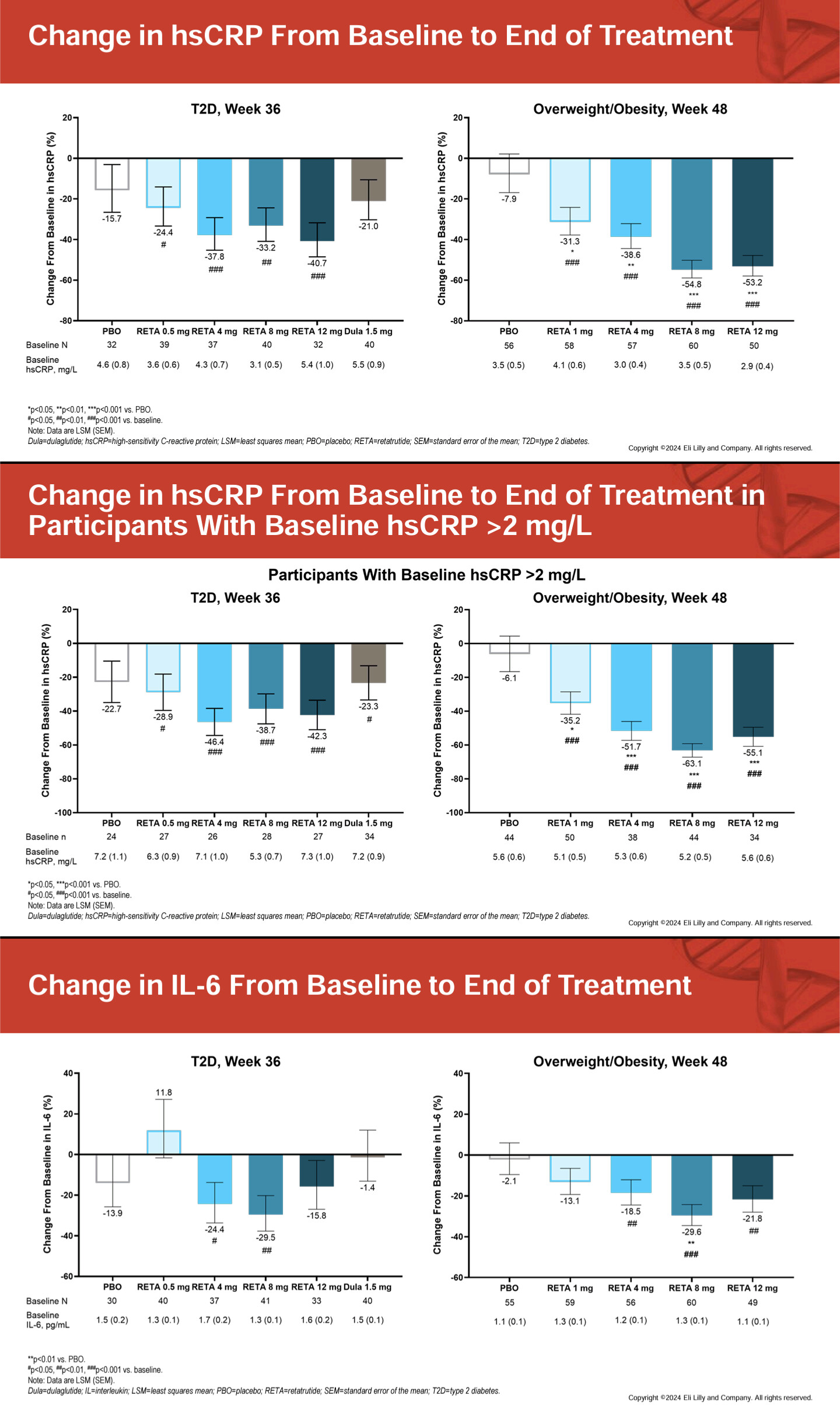
Tirzepatide treatment was associated with lower all-cause mortality compared to semaglutide (1.1% vs 1.7%, HR 0.65, 95% CI 0.58-0.73; p<0.001). Tirzepatide use also demonstrated reduced incidence of heart failure (4.1% vs 5.4%, HR 0.76, 95% CI 0.70-0.81; p<0.001), atrial fibrillation (2.3% vs 2.9%, HR 0.79, 95% CI 0.72-0.87; p<0.001), cardiogenic shock (0.3% vs 0.4%, HR 0.74, 95% CI 0.58-0.94; p=0.012), and acute kidney injury (3.5% vs 4.0%, HR 0.86, 95% CI 0.80-0.93; p<0.001). Similar benefits were observed for pulmonary embolism, pulmonary edema, and cerebrovascular disease.
A little under 6 months on retatrutide and it’s been eye opening to see how much some of my metabolic markers have improved :
- A1c: from 5.7 to 5.2%
- egfr (calculated ) : from 75 to 101. That’s a crazy number
- AST and ALT: from ast/alt ratio being a little over 1 to mess than 1
Can you please share the pre and post weights that drove those results over that time period?
Nothing earth shattering, about 9 pounds. I’m maybe 10 pounds from goal weight.
Also, recent weight loss puts me back at my lowest weight from a year ago. But back then A1c was 5.8%, and egfr was in the low 80s. So these metabolic improvements are not mediated by weight loss.
Very interesting. I will also be interesting to see if the improvements remain after you pause or stop the retratrutide. Please let us know, when you find out.
I don’t expect these improvements to hold up if discontinuing, frankly. I don’t see why they would. What would be interesting to see is if lower dosage (which would stop weight loss) would maintain these metabolic benefits.
I saw a recent post on X where people did maintain the weight when dropping to a lower maintenance dose on GLP1s. I wish I could track it down for you but I think it’s a good idea to keep a low maintenance dose in there to maintain the benefits.
Yes, although dosing for maintenance can be more complicated than for weight loss. Some are able to lower dose, others have to stay on the same dose. Some start at a lower dose and realize 6 months later they need to change it, because of weight loss or gain, etc.
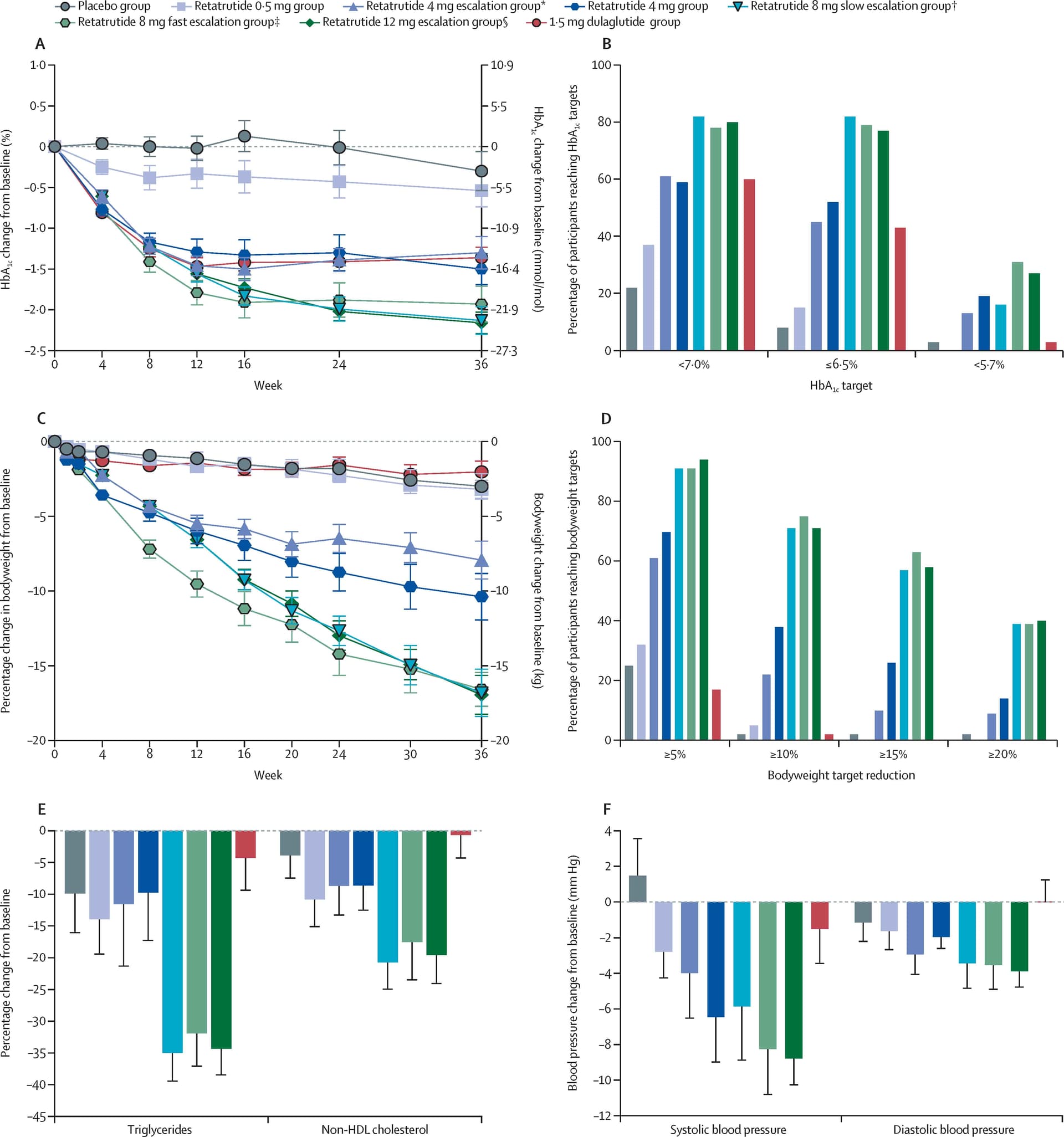
With retatrutide, the more interesting question is whether metabolic benefits are maintained if your weekly dose is less than 4mg. That’s because when looking the various improvements in metabolic markers, the minimum dose where these improvements happened was in the 4mg arm.
Do you happen a link to the study where the metabolic benefits began at 4mg?