Not where I live. Twice as much separately. Target Apob as low as you can get it. Mine is 68 going for 50. Attia is going for 30-40. You take cardiovascular disease off the table at these levels. i.e. no formation of plaque in arteries
Aspirin 150mg/day lowers Lp(a) by 56% after only 4 weeks?!?!?!?
That appears to be the finding of this clinical trial I stumbled across:
56% decrease in those with baseline Lp(a) greater than 25mg/dL. ![]()
It’s an older study (2007), but still paywalled. Maybe it’s a fluke, poorly done study with fabricated data for all I know, but if I could tolerate aspirin I’d certainly give this a 4 week before-and-after self-trial.
Here is more recent, updated and thorough review on this topic:
More recent, yes, but it’s a review and basically says “we need more data”. The point about Lp(a) being elevated at the time of an acute ischemic event is interesting, so hopefully that doesn’t explain the large decrease after aspirin therapy:
“There are a few small studies that have shown a quite significant Lp(a) lowering effect of aspirin therapy [18,19] with a wide range of effect size. These studies are limited due to their small size which makes it difficult to reduce confounding from a regression to the mean effect. In some cases, initial measurements were also taken at the time of an acute ischemic event which can lead to elevation in Lp(a) from baseline due to its role as an acute phase reactant [19]. Additional high-quality clinical studies are required to further evaluate this effect.”
Barring these small trials, we need to wait it out. Olpasiran and Zerlasiran data seems really promising. It will probably be 12 months before they get FDA approval. But all these trials enrolled for secondary prevention in patients who already had MACE. So for primary prevention, it remains to be seen how the prescription, insurance approval and authorization process will work.
I have tried Niacin, it doesnt really help.
I do take ASA; albeit 81 mg as opposed to 160 mg.
Effect of aspirin on lipoprotein(a) in patients with ischemic stroke.pdf (230.9 KB)
The article was cited 37 times in Google Scholar - so some impact
Have you ever wondered how long you have to stay on statins in order to start benefitting from primary prevention of MACE? It looks like 2.5 years. But apart from the study, for most of us the issue of course is not being on statins, as much as being on statins that get you to the therapeutic goal of ApoB/LDL level appropriate for your cumulative risk factor score.
This study is people 50-75 years of age, with at least 2.5 years of life left. Note: mortality benefit does not appear here.
Evaluation of Time to Benefit of Statins for the Primary Prevention of Cardiovascular Events in Adults Aged 50 to 75 Years
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2773065
“Conclusions and Relevance These findings suggest that treating 100 adults (aged 50-75 years) without known cardiovascular disease with a statin for 2.5 years prevented 1 MACE in 1 adult. Statins may help to prevent a first MACE in adults aged 50 to 75 years old if they have a life expectancy of at least 2.5 years. There is no evidence of a mortality benefit.”
Earlier use is better (with addition of French, for A.D.):
**Evidence supporting primary prevention of cardiovascular diseases with statins: Gaps between updated clinical results and actual practice
Preuves à l’appui des statines en prévention primaire des maladies cardiovasculaires : écarts entre les études cliniques et la pratique réelle**
https://www.sciencedirect.com/science/article/pii/S1875213614000278
Summary
The use of pharmacological lipid-lowering intervention in individuals with hypercholesterolaemia and known cardiovascular disease or diabetes/chronic kidney disease is well established. Current European Society of Cardiology guidelines recommend immediate initiation of drugs in adjunct to lifestyle intervention in these patients at high or very high cardiovascular risk. In these clinical settings, statins are generally chosen as the first-choice drug intervention, in consideration of the robust evidence showing a reduction in all-cause mortality and major adverse cardiac events (MACE). In contrast, primary prevention with statins, even in the subset of patients at high-risk of cardiovascular events, is not well implemented. This might be related to a lack of public awareness regarding the actual risk associated with prolonged exposure to high concentrations of low-density lipoprotein cholesterol (LDL-C) and uncertainties in the clinical evidence coming from the earliest trials in this patient subset. However, recent observational studies suggest that lowering LDL-C earlier in life and for a longer duration can substantially decrease the burden of cardiovascular disease and mortality. Moreover, results from recent well-conducted large meta-analyses of randomized clinical trials showed that primary prevention with statins reduced all-cause mortality by 14% and MACE by > 20% – findings similar to those observed for the use of statins in secondary prevention. Recently published American Heart Association/American College of Cardiology guidelines on the treatment of blood cholesterol emphasize that primary prevention using high-dose statins in individuals with LDL-C ≥ 190 mg/dL induces a benefit in atherosclerotic cardiovascular risk reduction that clearly exceeds the potential for adverse effects. We aim in this review to discuss the new data that advocate the use of statins in primary prevention earlier and more frequently, putting the efficacy evidence into perspective with new insights in terms of safety issues.
It’s an academically interesting question, for sure. But in practice, I think a simpler approach is that every single person should aim for an ApoB of <70mg/dl. If you can do that, you take the vast majority of CVD risk off the table, period. The area under the curve exposure model is very solid, and the earlier you start, the less risk you have.
CVD is the number 1 killer globally, by a long way. Given that statins and ezetimibe are extremely affordable nowadays, I see very few logical arguments against taking this approach at a broad level.
If individuals feel bad on statins, want to go “all natural” or whatever, that’s up to them to decide whether to live with higher ApoB, or look for alternative approaches/medications.
One of the major benefits of statins is lowering of inflammation which causes soft plaque to form from calcified plaque thus causing embolism
Do you know of a way to get it in the UK?
EDIT: if you’re young.
I tried with pharmacies and didn’t manage. I assume you need a prescription from a doctor saying that you are at high risk.
Yes, that’s the conclusion I came to. Private GP willing to prescribe and the pay £450 plus. Guess I’ll be waiting another 6 years.
If you go to Turkey, some pharmacies are OK with giving Shingrix. A friend of mine who’s 25yo did it. About €100 I think. Can be combined with holidays ![]()
Review 2022. Of interest: genetic factors and differential impact of SNPs. Other points of interest impact of ethnicity on statin and drug combinations, periodontitis, cholesterol dependent and independent effects.
Statins and cognition: Modifying factors and possible underlying mechanisms
"Recently, a meta-analysis showed that statins have more cognitive benefits in APOE4 carriers and patients with higher cholesterol levels (Xuan et al., 2020). Similarly, in APOE4 carriers, a longitudinal study has found that statin use was associated with a slower rate of global cognition decline over 6 years compared with non-users in community-dwelling elderly Australians age 70–90 years. Meanwhile, in non-APOE4 carriers, the rate of memory or cognitive decline in long-delayed recall performance was similar between statin users and non-users (Samaras et al., 2019). Also, it has been reported that while the users of statins showed an increased risk of AD, only APOE4-carrier statin users displayed a slightly lower risk for AD and dementia, especially in men (Dagliati et al., 2020). Nevertheless, autopsy evidence of statin users in autopsy-confirmed Alzheimer’s dementia brains did not demonstrate a significant difference in any AD pathological neuroimaging markers, suggesting that the statin use neither improves nor worsens AD pathology according to their APOE4 status (Crum et al., 2018).
Several studies have indicated that single nucleotide polymorphisms (SNPs) can alter the pharmacokinetics of statins (Smiderle et al., 2016; Naito et al., 2017; Ahangari et al., 2020). For example, the genetic variant of the cytochrome P450 (CYP) family genes, encoding for the main enzymes of the hepatic metabolism of statins, can alter body exposure to statins, making patients more vulnerable to their effect on cognitive function (Schultz et al., 2018). It has been reported that the CYP2C19 polymorphism (rs4388808) confers protection against the Aβ burden in AD patients (Benedet et al., 2018). Besides, the gene encoding for CYP2C9 is linked to familial AD. CYP enzymes are also responsible for the metabolism of certain antihypertensive drugs, which are most often concomitantly used in older adults (Barthold et al., 2020), suggesting that some combinations of statins and antihypertensive drugs may alter the activity of CYP enzyme family and subsequently have a various effect on Alzheimer’s disease and related dementias (ADRD) risk. Consequently, the inconsistency among studies regarding the effects of statins on cognitive performance or memory impairment could be explained by differences in drug metabolism and transport (i.e., pharmacokinetic interactions between certain statins with other drugs and certain genetic variations of CYP enzymes) (Barthold et al., 2020). The effect of CYP polymorphisms on the cognitive effects of statins should be considered in future clinical trials as they can mask the outcomes of the analysis.
Moreover, the HMGCR gene polymorphisms can influence both the cholesterol-lowering response to statin and the pleiotropic statin protective effect on cognitive function (de Oliveira et al., 2022). It has been reported that rs3846662 might increase the HMGCR expression and thereby contribute to the onset and progression of AD (Ma et al., 2019). Conversely, rs17238484 was associated with a minor reduction in the risk of AD (Hon-Cheong et al., 2017). Additionally, a study conducted on three cohorts evaluated the association between AD and the HMGCR’s rs3846662 G negative status and highlighted that this variant was one of the most important protective genetic factors for AD, behind APOE2 (Leduc et al., 2015). However, Mendelian randomization analyses led on HMGCR did not suggest that the use of statins could alter AD risk (Williams et al., 2020). Finally, a pilot study showed that the genetic variants of CETP (Cholesteryl Ester Transfer Protein), rs5882-AA, and, the genetic variant of NR1H2 (Nuclear Receptor subfamily 1 group H member 2), rs2695121-CC, were associated with cognitive dysfunction, especially in patients using lipophilic statins. However, the effect of rs3846662 (HMGCR variant) had not been able to be evaluated (de Oliveira et al., 2022). More studies are needed to elucidate the exact effect of HMGCR gene polymorphisms in statin users."
It just doesn’t make any sense that statins would affect cognitive performance.
It’s to begin with determined by 10,000 genes all with very small effects, by definition a few genes nuking LDL-C by PCSK9 or HMGCR won’t have much of any pleiotropic effect on cognitive performance.
At the same time a large meal of chili or getting a $10 bonus on a task will have a large effect, so maybe the genetic studies won’t tell us much…
And this will have a large effect as well…
Most statins increase the risk of diabetes so of course they could affect cognitive performance.
Analysis from 2018.
Adverse effects of statin therapy: perception vs. the evidence – focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract
Single injection can reduce LDL by up to 69% for life. Not peer reviewed as yet.
“Among the 14 participants, a maximum LDL-C reduction of 69% was achieved in a participant in the 0.6 mg/kg cohort.”
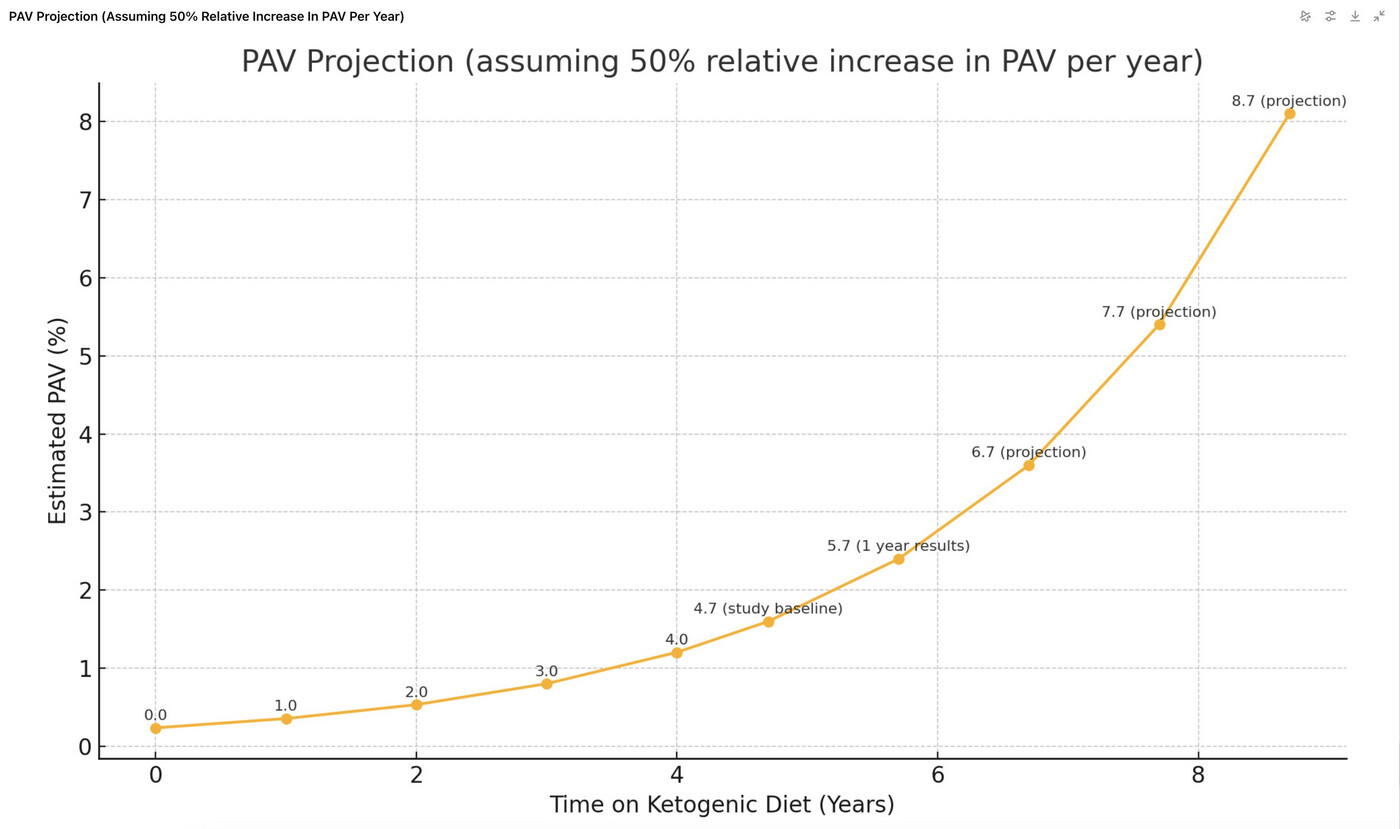
Your heart or areas adjacent to brain will literally explode of plaque if you go on a KETO diet and have untreated high cholesterol. Exponential growth.

https://x.com/theproof/status/1927108848747225129#m
Since “Plaque Begets Plaque”, (and ApoB as well), it’s a superexponential (growth rate increasing faster over time).
That is a naughty chart as it uses projection assuming an accelerating rate of increase. I have no axe to grind here, but I don’t like that sort of approach. At least they could use a different colour and we don’t necessarily know the source of the original figures prior to the study as well.