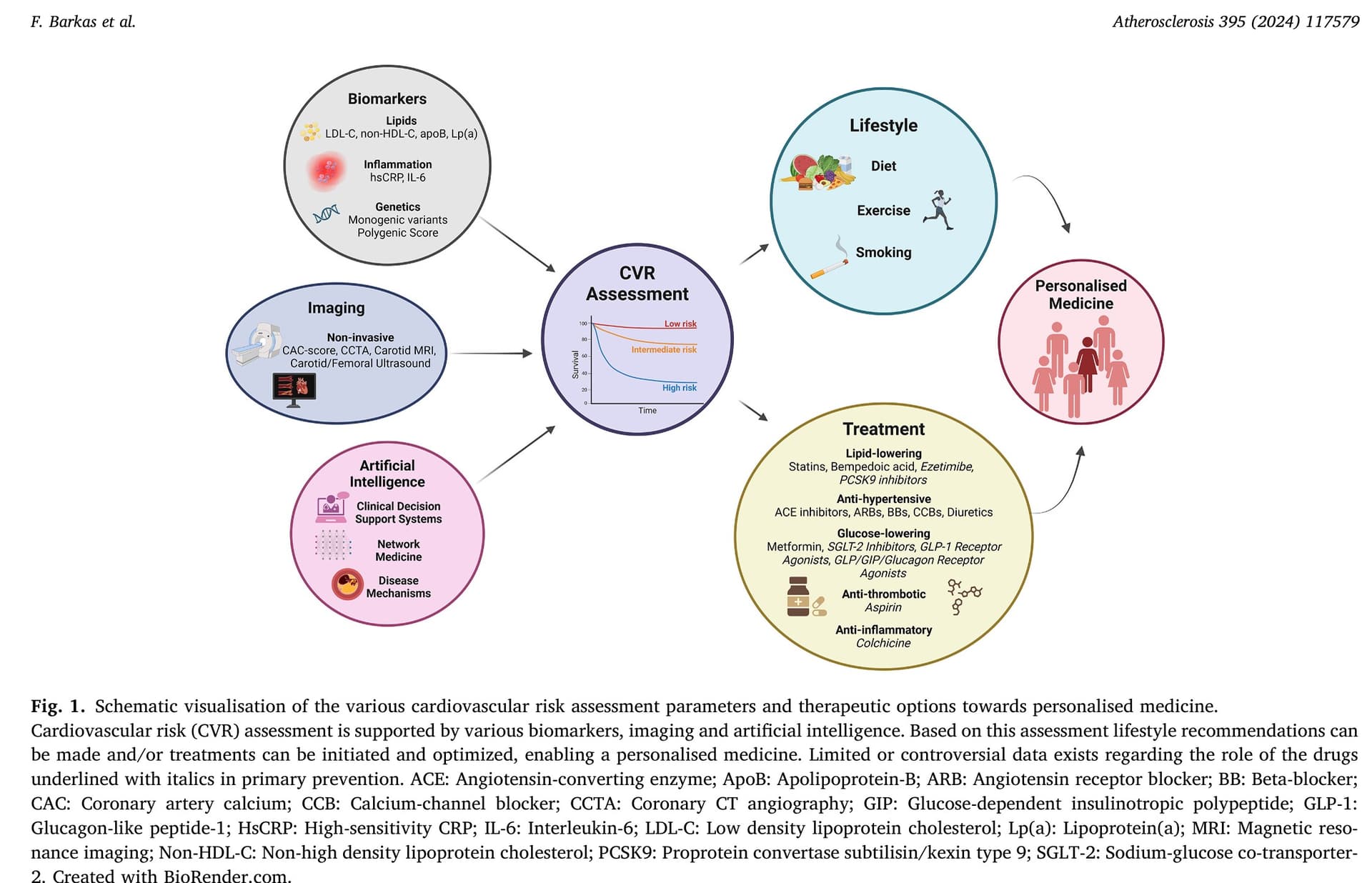
Advancements in risk stratification and management strategies in primary cardiovascular prevention 2024
This paper sums up the “mainstream” view:
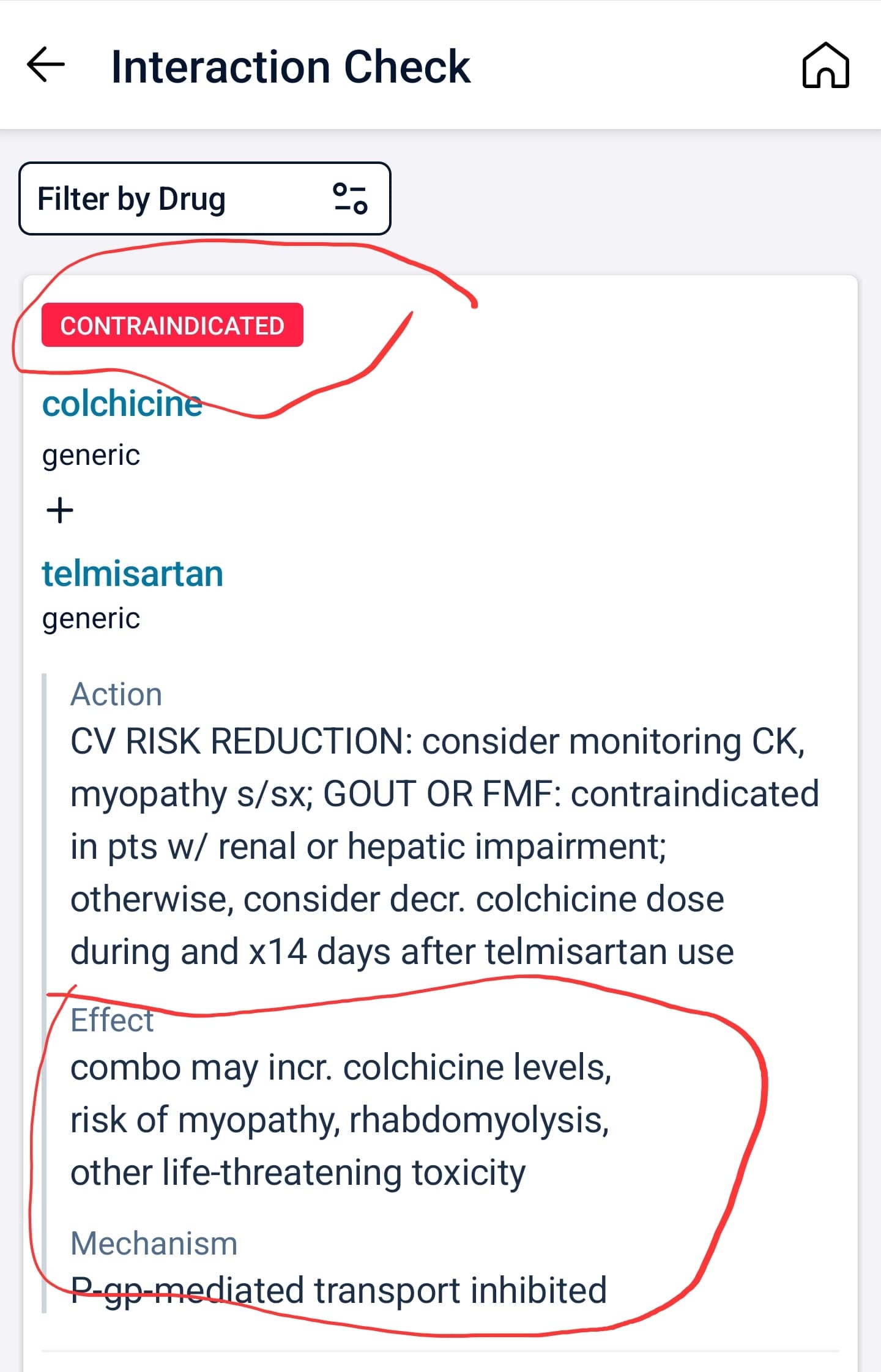
For me the post interesting part is 4.2.5 Anti-inflammatory drugs:
There is increasing evidence regarding the relationship between the use of immunomodulating drugs and favourable cardiovascular outcomes, mainly in patients with established ASCVD. The first anti-inflammatory therapy approved for secondary ASCVD prevention is low-dose colchicine, which is a broad anti-inflammatory drug. Trials like COLCOT and LoDoCo2 have demonstrated reduced MACE in CAD patients treated with low-dose colchicine compared with those on placebo. In this setting, colchicine may also promote plaque stabilisation. A recent study has demonstrated reductions in LAPV, a measure of plaque stability, as assessed by CCTA in patients receiving colchicine on top of optimal treatment compared to those receiving standard-of-care treatment; of note, overall atheroma plaque volume was not different between groups. According to current guidelines low-dose colchicine (0.5 mg daily) may be considered for secondary prevention especially in patients with recurrent ASCVD events under optimal medical therapy or insufficiently controlled risk factors after acute coronary syndrome episodes. However, data on the impact of colchicine use on primary prevention is still lacking. Similarly, while methotrexate and canakinumab have shown promising results in secondary ASCVD prevention, their efficacy remains uncertain, and benefit may be restricted to selected patients such as patients with heightened inflammation (hsCRP>2 mg/ml) or TET2-driven CHIP. The ongoing ZEUS trial (NCT05021835) investigating IL-6 inhibition in ASCVD patients with CKD may provide further valuable insights into immunomodulatory approaches for ASCVD management
And mixed results on omega 3:
Another potential strategy is to use 3-polyunsaturated fatty acids (3-PUFA, omega-3 fatty acids), particularly the highly purified, stable eicopentaeonoic acid (EPA)-derivative icosapent ethyl. However, it is noteworthy that while the REDUCE-IT trial demonstrated cardiovascular benefits, these benefits were not statistically significant in the primary prevention subgroup. Conversely, the STRENGTH trial failed to show a significant reduction in MACE among high-risk patients treated with EPA, leading to a downgrading of the recommendation for EPA use to class IIb in the 2021 ESC prevention guidelines. On the other hand, several studies have reported plaque regression or increased stability following EPA use in patients with coronary atherosclerosis
So plaque regression does not lead to MACE reduction?