I would assume myself that a smaller dose of TRT might keep the HCT down. I don’t myself use TRT, but I do have a high SHBG that I don’t worry about.
Food for thought regarding what I said earlier about living at extreme altitude. That’s also secondary erythrocyteosis. We can speculate on whether it’s the same or different from from a red blood cell from testosterone (or an SGLT2), but I think it’s massively important.
Of course, this does not apply to people who have other risk factors in addition to high hematocrit.
2023 study: Preparing to download ...
AI analysis:
What Was the Study About?
• The researchers examined whether Andean highlanders who develop excessive erythrocytosis (EE+)—a condition marked by abnormally high red blood cell counts—tend to have blood that clots more easily, increasing their risk of thrombosis.
• They compared this group (EE+) to highlanders without this condition (EE–) and to people living at sea level (lowlanders, LL).  
⸻
Key Findings (Explained Simply)
1. Surprisingly, EE+ individuals had blood that was less prone to clotting:
** • Using a technique called rotational thromboelastometry, researchers found that EE+ participants took longer to form clots and formed weaker ones than EE– and LL groups. This means their blood was actually hypocoagulable, not hypercoagulable. **
2. When haematocrit was normalized to 40%, some clotting function improvements were seen:
• In tests called FIBTEM, the clot firmness returned to normal.
• However, in EXTEM and INTEM tests, clot firmness stayed weaker than normal, suggesting platelet function is part of the issue. 
3. All other clotting measures looked normal:
• Factors like thrombin generation, standard clotting proteins, and coagulation inhibitors were similar across all groups.  
• Moreover, none of the highlanders (EE+ or EE–) reported any history of blood clots forming in their veins. 
⸻
What’s the Bottom Line?
• Main takeaway: Highlanders with excessive red blood cell counts (EE+) do not have an increased tendency for blood clots—in fact, their blood clotting appears reduced in certain conditions.
• Though their plasma clotting factors are normal, the altered clotting behavior points toward platelet abnormalities being at play.
• Overall, the study suggests that EE+ individuals do not face a heightened risk of thrombosis.
⸻
Why This Matters
• Prior to this research, it wasn’t clear whether having extra red blood cells (as seen in chronic mountain sickness) increased the risk of clot-related problems.
• These findings help clarify that, at least in the conditions studied, excessive red blood cells don’t automatically translate to more clotting risks.
• Understanding how platelets behave under extreme high-altitude conditions may offer insights into coagulation and health in similar hypoxic environments.
This might require a dedicated thread on TRT and optimal HCT to stay focused here on SGLT2i? (although of course the topics are intertwined for those taking both)
I agree. I feel bad cluttering up the thread.
Interesting SGLT2i are not considered performance enhancing. I would think with the epo stimulation and higher Hct that they would be banned from sports events. Perhaps they are and they don’t have to be considered performance enhancing?
Results The cohort consisted of 152 591 new users of antidiabetic drugs, with 15 125 SGLT-2i users, 31 896 DPP-4i users, 15 723 other second-line to third-line antidiabetic drug users and 89 847 first-line antidiabetic drug users at cohort entry. After adjusting for all relevant confounders, SGLT-2i use was associated with a reduced rate of all-cause mortality compared with metformin monotherapy (HR: 0.77, 95% CI: 0.64 to 0.93) or DPP4-i (HR: 0.57, 95% CI: 0.51 to 0.63). This reduced rate of all-cause mortality appeared to be independent of sex and cardiovascular disease.
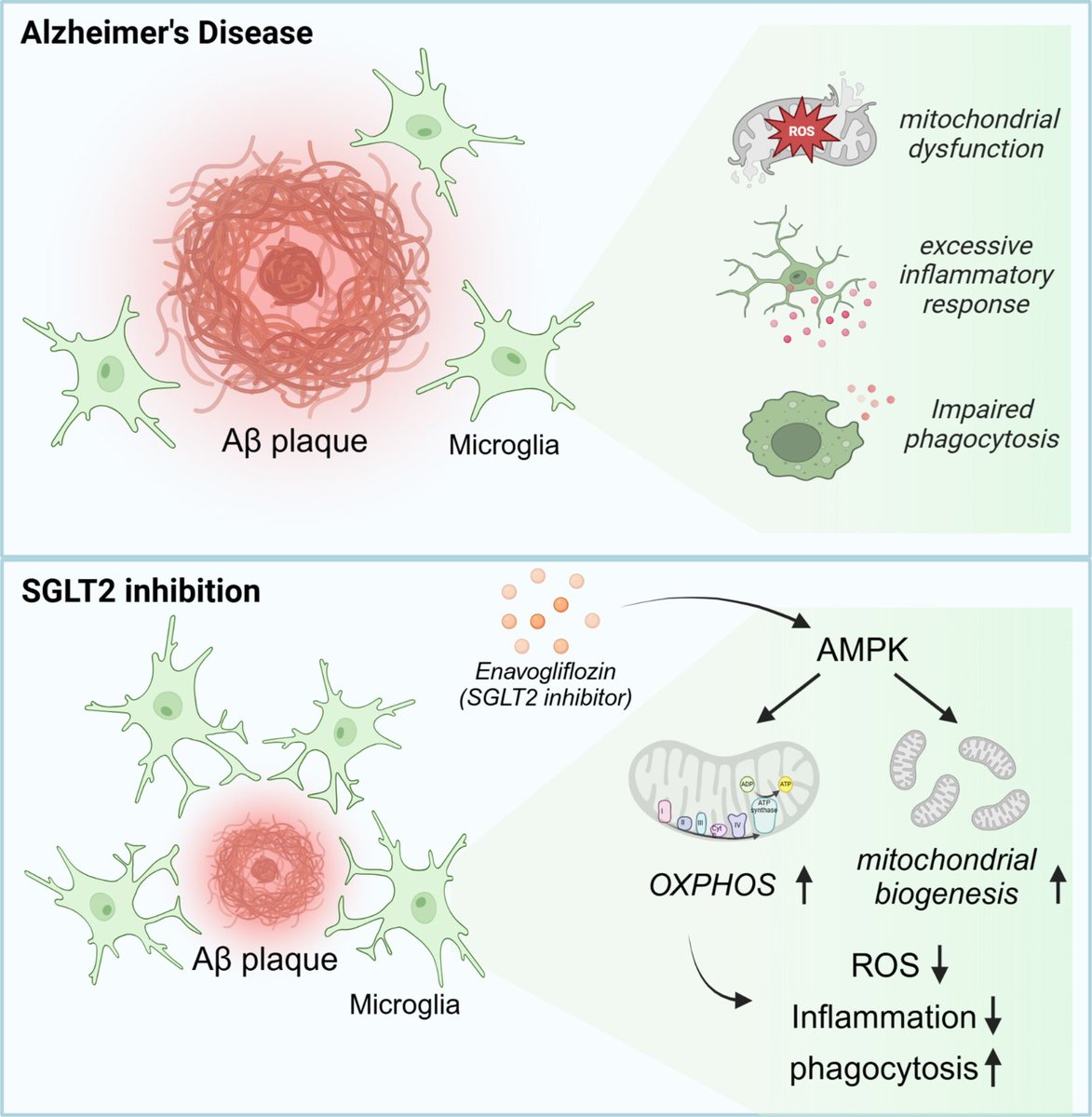
I’m always suspect of mouse models of Alz, but here it is, given that caveat:
Open Access Paper:
SGLT2 Inhibition by Enavogliflozin Significantly Reduces Aβ Pathology and Restores Cognitive Function via Upregulation of Microglial AMPK Signaling in 5XFAD Mouse Model of Alzheimer’s Disease
These findings highlight the multifaceted neuroprotective effects of SGLT2 inhibition in AD, demonstrating its potential to mitigate pathology and improve cognitive function. By uncovering its impact on neuroinflammation and microglial function, this study establishes SGLT2 inhibition as a promising therapeutic avenue for AD and other neurodegenerative disorders.
Pharmacokinetics and Tissue Distribution of Enavogliflozin in Mice Using a Validated Liquid Chromatography–Tandem Mass Spectrometry Method
https://www.mdpi.com/2076-3417/15/3/1445
“In multiple administrations, plasma exposure of enavogliflozin and urinary glucose excretion (UGE) over 24 h showed a clear pharmacokinetic and pharmacodynamic correlation in the dose range of 0.1–2.0 mg [11,13]. Additionally, as a result of high and sustained kidney distribution [7], enavogliflozin demonstrated long-lasting UGE up to 168 h and equivalent daily UGE compared with dapagliflozin 10 mg [11]. These results suggest the greater potency and long-lasting efficacy of enavogliflozin compared to dapagliflozin, which may reflect the importance of kidney distribution of in the pharmacodynamics of these SGLT2 inhibitors. Although about 2 years have passed since its market approval, a meta-analysis of enavogliflozin was performed using four clinical trials that included 684 T2DM patients for clinical outcomes over 12–24 weeks of clinical use. As a result, enavogliflozin is a well-tolerated and effective SGLT2 inhibitor for T2DM and could be superior to dapagliflozin with regard to hemoglobin A1c levels below 7.0% over 6 months of clinical use [11,14,15,16]”
Urine Glucose Excretion Calculator:
Old conversation, but it’s time for a partial answer. I have been off rapamycin for months now. I was also off dapagliflozin for 2 months until two weeks ago when I added 5mg back in.
So far, I detect zero impact on my resistance training progression or my ability to gain weight (I’m gaining a little more than 1 lb/wk). I don’t plan on adding rapamycin anytime soon, but when I do, I’ll comment again.
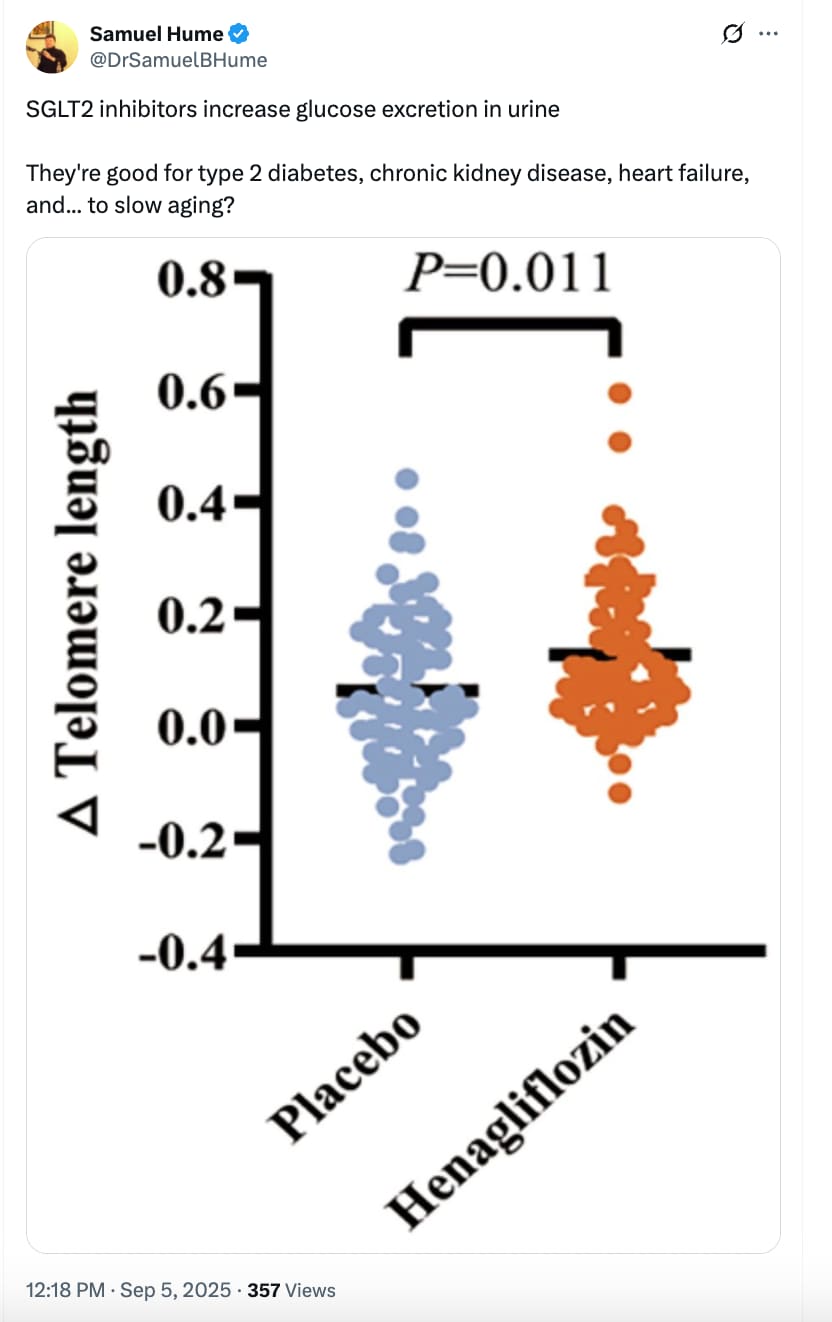
More good news on SGLT2 inhibitors:
Highlights
• Henagliflozin can extend telomere length
• Henagliflozin affects the insulin-like growth factor-1 system and immune cell function
• Henagliflozin can lead to changes in various metabolites
• This clinical trial demonstrates the anti-aging potential of SGLT2i
Full Paper (open access)
Effect of henagliflozin on aging biomarkers in patients with type 2 diabetes: A multicenter, randomized, double-blind, placebo-controlled study
https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(25)00404-5
AI Summary:
Here’s a detailed summary of the attached Cell Reports Medicine paper:
Citation
Zhang et al. (2025). Effect of henagliflozin on aging biomarkers in patients with type 2 diabetes: A multicenter, randomized, double-blind, placebo-controlled study. Cell Reports Medicine, 6:102331. https://doi.org/10.1016/j.xcrm.2025.102331
Background
- Type 2 diabetes mellitus (T2DM) is closely tied to accelerated aging, driven in part by cellular senescence and chronic inflammation.
- SGLT2 inhibitors (SGLT2i), widely used for glucose control and cardiorenal protection, are thought to act as caloric restriction mimetics, raising the possibility of anti-aging benefits.
- Preclinical studies suggested lifespan extension and reduced senescence burden with SGLT2i, but clinical data on aging biomarkers were lacking.
Study Design
- Design: Multicenter, randomized, double-blind, placebo-controlled trial.
- Participants: 150 patients with T2DM (142 completed).
- Intervention: Henagliflozin 10 mg/day vs. placebo for 26 weeks.
- Primary endpoint: Change in telomere length (peripheral blood leukocytes).
- Secondary endpoints: IGF-1/IGFBP-3 system, glucose metabolism, β-hydroxybutyrate, immune function, metabolomics.
- Trial registration: ChiCTR2300068127.
Key Findings
1. Telomere Length
- Significant increase in telomere length in henagliflozin vs placebo (mean difference: +0.06 units; p = 0.011).
- 90.5% of treated patients showed telomere lengthening vs. 65.6% with placebo.
2. IGF-1 / IGFBP-3 Axis
- IGFBP-3 levels increased significantly (p = 0.013).
- IGF-1 and IGF-1/IGFBP-3 ratio tended lower (not statistically significant).
- Suggests a possible downregulation of GH/IGF-1 signaling, a pathway linked to lifespan extension.
3. Metabolic Outcomes
- Improved glucose metabolism: lower fasting plasma glucose (−1.92 mmol/L vs −1.17 mmol/L; p = 0.030) and HbA1c (−1.37% vs −1.04%; p = 0.047).
- Increased β-hydroxybutyrate (+0.05 mmol/L; p = 0.002).
- Reduced body weight, BMI, and serum uric acid compared with placebo.
4. Immune Function
- Granzyme B expression in cytotoxic T lymphocytes (CTLs) increased significantly (p = 0.033).
- Trends toward higher perforin expression in CTLs and T cells.
- No significant changes in inflammatory cytokines (IL-6, IL-10, IFN-γ).
- Suggests improved immune cell cytotoxic function (partial reversal of immunosenescence).
5. Metabolomics
-
Untargeted serum analysis identified 53 altered metabolites after 26 weeks.
-
Key changes:
- ↑ Thiamine levels and enhanced thiamine metabolism
- ↓ Phosphatidylcholine (PC), phosphatidylethanolamine (PE), sphingosine
-
These shifts point toward improved energy metabolism and reduced immunosuppressive lipid species.
6. Safety
- Well tolerated.
- Adverse events balanced across groups.
- Serious AEs occurred only in placebo group.
- No diabetic ketoacidosis (DKA) reported.
Discussion
- Telomere extension is the first direct clinical evidence that an SGLT2i may influence an aging hallmark in humans.
- The IGF-1 axis modulation, ketone body increase, and immune enhancement parallel known effects of caloric restriction.
- Thiamine metabolism emerged as a novel pathway, suggesting possible mitochondrial/energy-related anti-aging mechanisms.
- Results align with previous SGLT2i benefits in cardiovascular, renal, and frailty outcomes in older populations.
- Potential role as multi-system geroprotective agents, possibly complementary to GLP-1R agonists.
Limitations
- Short duration (26 weeks) – no data on sustainability after discontinuation.
- Single dose of henagliflozin studied.
- Small immune-function sample size due to processing constraints.
- Telomere length is a useful but imperfect biomarker of biological aging.
- No functional clinical aging endpoints (frailty, cognition, physical performance) assessed.
Conclusion
Henagliflozin (10 mg/day for 26 weeks) in patients with T2DM:
- Extended telomere length,
- Improved glucose metabolism and ketone production,
- Modulated IGF-1/IGFBP-3 system,
- Enhanced cytotoxic T cell function,
- Induced favorable metabolomic shifts (↑ thiamine metabolism, ↓ sphingolipids).
This trial provides the first randomized clinical evidence of anti-aging biomarker modulation by an SGLT2 inhibitor, supporting their potential as geroprotective therapies.
=================================
Here’s a methods-first critique of the henagliflozin trial, focused on how convincingly it supports an “anti-aging” claim.
Bottom line
The study is a well-run, double-blind RCT that shows henagliflozin (10 mg/day, 26 weeks) favorably shifts several aging-adjacent biomarkers—notably leukocyte telomere length (primary endpoint), IGF axis components (↑IGFBP-3), ketones (↑β-hydroxybutyrate), selected immune cytotoxic markers (↑granzyme B in CTLs), and metabolomic pathways (↑thiamine metabolism; ↓PC/PE/sphingosine). However, the trial is short, single-dose, biomarker-only, with small mechanistic sub-samples and several analytic/methodologic caveats (surrogate outcomes, leukocyte composition, multiplicity, and untargeted ‘omics overfitting risk). The evidence is promising but preliminary for geroprotection; it does not yet demonstrate slowed biological aging or improved aging phenotypes.
Major strengths
- Gold-standard design for pharmacology: multicenter, randomized, double-blind, placebo-controlled; 150 randomized (142 analyzed), balanced baseline characteristics.
- A priori primary endpoint met: telomere length increased vs placebo (mean Δ difference ≈ +0.06; p = 0.011). Authors also re-checked the effect adjusting for smoking.
- Convergent biological signals: IGF axis shift (↑IGFBP-3), metabolic changes consistent with SGLT2i/CR-mimetic physiology (↓FPG, ↓HbA1c, ↑β-hydroxybutyrate), immune cytotoxicity signal (↑granzyme B in CTLs), and coherent metabolomic pathway enrichment (↑thiamine metabolism; ↓immunosuppressive lipids). These point in the same directional hypothesis.
- Safety profile: AE rates balanced; SAEs occurred only in placebo; no DKA reported.
Key limitations / potential biases
1) Surrogate focus and interpretability
- Telomere length was measured in peripheral blood leukocytes and reported in relative units (qPCR-style), not base pairs nor calibrated to biological age models; no direct mapping to “years of aging” is possible. Short-term (26 wk) TL changes can reflect leukocyte subset shifts as much as true telomere elongation/attrition dynamics. While global WBC/lymphocyte/monocyte counts are shown (no between-group differences in changes), granular cell-composition adjustments (e.g., neutrophils, naïve/memory T, B, NK subsets) are not modeled for the TL endpoint. This tempers claims that the TL rise reflects slowed cellular aging.
2) Multiplicity and small mechanistic samples
- Multiple secondary/exploratory endpoints (IGF axis, cytokines, immune cytotoxic markers, metabolomics) were tested; no formal multiplicity correction is reported. Positive immune signals derive from a small subset (n≈ 15 placebo, 19 active), and metabolomics from 56 participants (32 active, 22 placebo) with 53 metabolites declared different at p < 0.05—raising false-positive risk without false discovery rate control. OPLS-DA is susceptible to overfitting if cross-validation/permutation testing isn’t rigorous (not detailed in the excerpt). Conclusions about mechanisms should be cautious.
3) Short duration and single dose
- 26 weeks, single 10 mg dose; no durability or dose-response. No post-discontinuation follow-up to test persistence or reversal of biomarker changes.
4) Aging outcomes not assessed
- No clinical aging phenotypes (frailty index, gait speed, grip strength, cognitive measures), no composite biological age clocks (e.g., GrimAge/PhenoAge), and no organ-specific function beyond routine metabolic labs. The study demonstrates biomarker modulation, not extended healthspan.
5) Missingness and measurement constraints
- Body composition was analyzed in only n=22 due to equipment limits; lipid and blood-cell data have some missingness from site errors. The telomere assay and immune phenotyping required fresh blood at multiple centers—pre-analytical variability can creep in despite blinding. Details of TL assay reproducibility (intra-assay CV, calibrators) are not in the excerpt.
6) Generalizability and sponsorship
- Middle-aged T2DM cohort (mean ~52 y, China); unclear generalizability to older (>65), non-diabetic, or multi-morbid populations. The study drug was supplied by the manufacturer (no role in conduct per authors), which is standard but still worth noting in early-phase biomarker work.
Statistics & interpretation notes
- Primary analysis compares change-from-baseline between arms using t/Mann-Whitney tests; ANCOVA with baseline adjustment typically offers better precision and is often preferred for continuous endpoints. Authors did a smoking-adjusted sensitivity for TL; broader covariate-adjusted models could help.
- IGF-1/IGFBP-3 ratio trended lower but was not significant; anchoring mechanistic claims (GH/IGF-1 suppression) on a non-significant ratio is suggestive, not confirmatory.
- Improvements in glycemia, BMI, and uric acid plausibly mediate favorable biomarker shifts; whether henagliflozin’s effects exceed what would be expected from any agent achieving similar metabolic changes remains untested here (no active comparator).
How convincing is the “anti-aging” claim?
- Supportive: Pre-specified primary endpoint improved; multiple orthogonal biomarkers shifted in a direction consistent with CR-mimetic physiology; safety acceptable.
- Not yet definitive: Lacks clinical aging endpoints, robust cell-composition controls for TL, multiplicity control for ‘omics/immune panels, longer follow-up, and dose-response. The findings are best framed as biomarker-level evidence of geroscience plausibility, not proof of slowed aging or improved healthspan.
What would strengthen the evidence (actionable next steps)
- Longer, multi-dose, multi-center RCTs with post-drug follow-up to test durability/disease-agnostic benefits.
- Add epigenetic clocks (GrimAge, PhenoAge, DunedinPACE), proteomic clocks, and functional aging measures (frailty index, gait speed, grip strength, cognition).
- TL measurement upgrades: flow-FISH or absolute TL (bp) calibration; explicit adjustment for leukocyte subsets (full differential and lymphocyte sub-phenotyping) in TL models.
- Multiplicity control (FDR) and pre-registered analysis plans for ‘omics and immune panels; cross-validated OPLS-DA/permutation testing.
- Active-comparator arms (e.g., GLP-1RA) or combination to test class-specific vs shared metabolic effects on aging biomarkers.
Overall verdict
A carefully executed, hypothesis-generating RCT that advances the field by showing consistent biomarker modulation in humans on an SGLT2 inhibitor. It provides encouraging, not yet conclusive evidence for anti-aging potential. Translation to clinical geroprotection will require longer, larger, and function-focused trials with rigorous biomarker methodology and multiplicity control.
There’s a new gliflozin every time I visit this page!
E.g. kind of hard to believe that Canagliflozin, Ganagliflozin, and Janagliflozin are all real drugs.
Is there anything unique about henagliflozin compared to all the others?
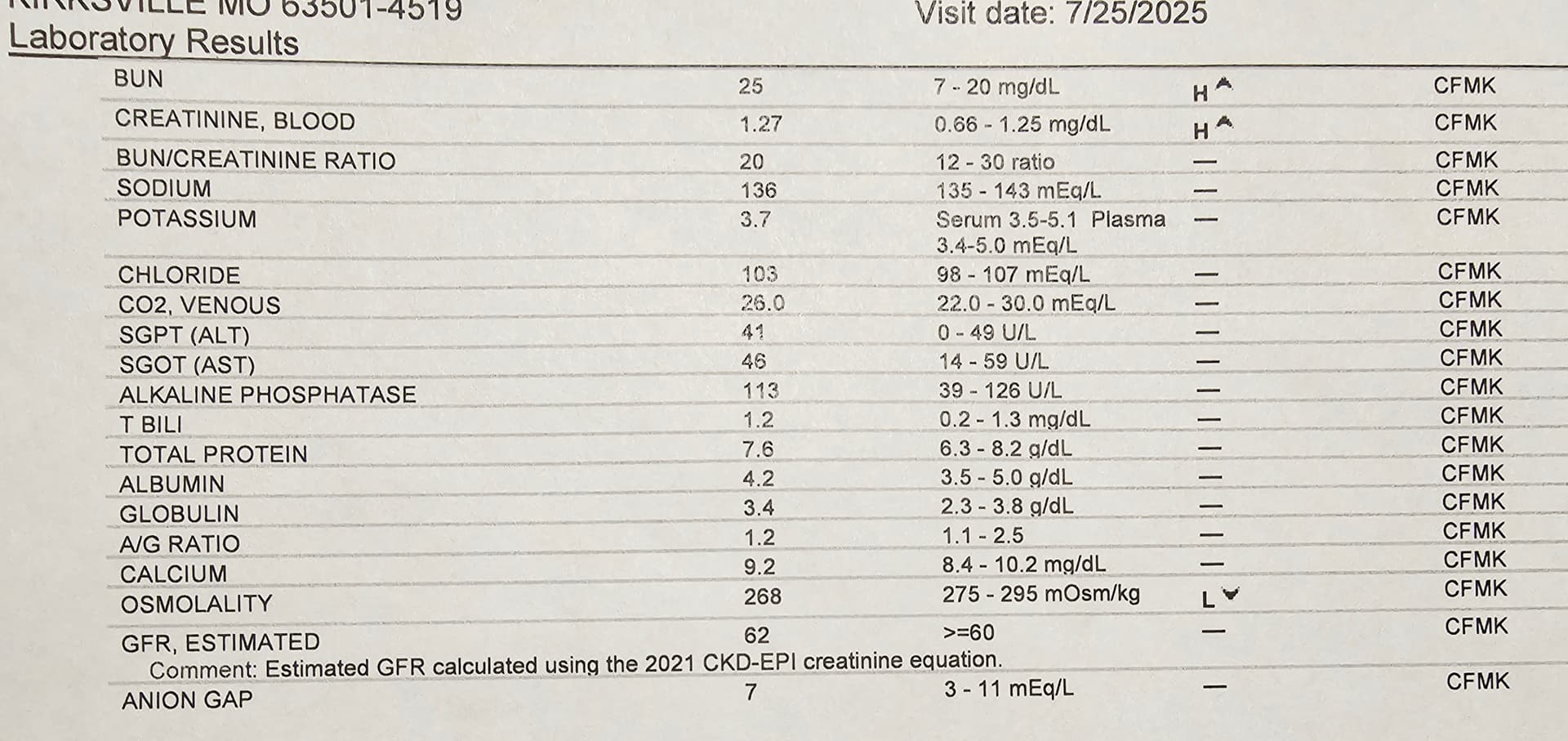
Based on the discussions, research and reports I found here on SGLT2i inhibitors for longevity, I was curious to give it a try… and consulted with my personal physician. He agreed it definitely could be helpful… my GFR at 62 at my end of July blood work… is just passable, maybe a SGLT2i could push me up.
Was totally gung-ho… but had sticker shock on Jardiance self pay about $900 per month. ![]()
My physician filed with my health insurance for kidney health. They approved with a co-pay of $60 per month. Much better. My pharmacist said Jardiance has a helping site for a discount to just co-pay $10 per refill. A few questions… nothing really medical. Odd. I got the discount. From $900 monthly to $10 monthly. Crazy.
If you can get your physician to sign off… even if insurance won’t cover it all. Go to this link for $10 co-pay: https://patient.boehringer-ingelheim.com/us/products/jardiance/type-2-diabetes/savings
Jardiance is the brand name for the prescription drug empagliflozin, an oral medication used to treat type 2 diabetes, heart failure, and chronic kidney disease in adults. It is part of a class of drugs known as sodium-glucose cotransporter 2 (SGLT2) inhibitors.
How Jardiance works
Jardiance works by targeting the SGLT2 proteins in the kidneys. SGLT2 is responsible for reabsorbing glucose (sugar) from the urine back into the bloodstream. By blocking this protein, Jardiance increases the amount of glucose that is excreted in the urine, which helps lower blood sugar levels. This process can also lead to modest weight loss.
I have been on Jardiance for a few weeks… no issues… I seem to pee just a bit more often… that’s it.
I am looking forward to seeing my GFR score in 6 months
Here is an ai summary
Here’s your updated table, now including henagliflozin (SHR-3824), with all key molecular target metrics aligned in one place:
| Drug | Primary target(s) | SGLT2 IC₅₀ (nM) | SGLT1 IC₅₀ (nM) | Selectivity (SGLT2 over SGLT1) | Notable notes / secondary targets |
|---|---|---|---|---|---|
| Canagliflozin | SGLT2 » SGLT1 | 4.4 | 684 | ~155× | More SGLT1 cross-inhibition; also inhibits SGLT6. |
| Dapagliflozin | SGLT2 » SGLT1 | 1.6 | 803 | ~502× | Highly selective; minimal SGLT1 inhibition clinically. |
| Empagliflozin | SGLT2 » SGLT1 | 3.1 | 8,300 | ~2,680× | Among the most SGLT2-selective. |
| Ertugliflozin | SGLT2 » SGLT1 | 0.877 | 1,960 | ~2,235× | Very potent and highly selective. |
| Bexagliflozin | SGLT2 » SGLT1 | 2.3 | 5,600 | ~2,435× | Highly selective. |
| Ipragliflozin (JP) | SGLT2 » SGLT1 | 7.38 | 1,876 | ~254× | Moderate selectivity. |
| Luseogliflozin (JP) | SGLT2 » SGLT1 | 2.26 | ~3,990 | ~1,765× | ~1,770-fold selectivity reported. |
| Tofogliflozin (JP) | SGLT2 » SGLT1 | 2.9 | 8,444 | ~2,912× | Strong human and rat selectivity. |
| Remogliflozin etabonate (IN) | SGLT2 » SGLT1 | ~14 | ~1,100 | ~79× | Least selective; also inhibits SGLT5. |
| Sotagliflozin (dual) | SGLT2 & SGLT1 | 1.8 | 36 | ~20× | Intentionally dual-action; gut SGLT1 inhibition prominent. |
| Henagliflozin (CN) | SGLT2 » SGLT1 | 2.38 | 4,324 | ~1,818× | Highly selective; ≥800-fold SGLT2 vs SGLT1 selectivity reported. |
Notes on Henagliflozin
- Origin & Approval: Henagliflozin (SHR-3824) is developed in China and approved there for treating type 2 diabetes.
- Selectivity: In vitro data indicate IC₅₀ ≈ 2.38 nM (SGLT2) and ≈ 4,324 nM (SGLT1), yielding a selectivity ratio of roughly 1,800-fold. Some reports simplify this as “≥ 800-fold” selectivity.
Summary Insights
- Most SGLT2-selective: Empagliflozin, ertugliflozin, bexagliflozin, tofogliflozin—all showing high selectivity (≥ ~2,200×).
- Henagliflozin fits strongly into this high-selectivity group (~1,800×), similar to luseogliflozin (~1,700×).
- Lower selectivity: Canagliflozin and remogliflozin have much less SGLT1 selectivity, which may influence GI glucose absorption and side effect profiles.
- Dual SGLT1/2 blocker: Sotagliflozin (~20×) intentionally targets both transporters—and is the least selective in the list.
Let me know if you’d like more detail on secondary targets, assay methodologies, or how these differences impact pharmacodynamics or clinical outcomes like heart failure or renal protection.
Did you ever get your cystatin C checked? If not, you are probably worrying about your GFR for no reason. If you take creatine monohydrate and lift weights, your creatinine-based GFR is going to be falsely decreased. Cystatin C should give you a much better GFR estimate.
That being said, IMO Jardiance is great to add to a longevity/health stack IF your hematocrit doesn’t get too high. Testosterone and SGLT2i both raise hematocrit, also GH and GH-releasing peptides can raise it as well.
I threw my blood test results (pdf) into ChatGPT and asked it to compare my blood levels with what are considered “optimal levels” based on Peter Attia’s medicine 3.0 standards. Very interesting results. I encourage others to do the same.
Your eGFR is low according to Attia:
Renal Function
- eGFR: 67 mL/min/1.73 m² — While “normal” is >59, Attia’s optimal is ≥ 90 for healthy adults without kidney disease.
Periodic reminder that labcorp cystatin c is garbage, get tested at quest.
I’ve switched all my blood work from LabCorp to Quest. At least in my area, the difference in service is night and day better w/Quest. LabCorp is overbooked, understaffed, and getting my results is like pulling teeth. Quest automatically releases each test result as it comes in, whereas LabCorp waits until ALL results have come in before releasing anything unless you go to their website and request special permission EVERY TIME to get “preliminary results”. ![]()
Considerations when choosing an SGLT2 inhibitor:
for day-to-day glucose control, dual SGLT1/2 (e.g., sotagliflozin) hasn’t proven superior to highly selective SGLT2 inhibitors on A1c, though it does blunt post-meal spikes more. Here’s what the evidence shows:
- A1c lowering with SGLT2 inhibitors (class effect): Meta-analyses of approved SGLT2i show mean A1c drops ≈0.5–0.7% vs placebo; small differences among individual drugs, with canagliflozin 300 mg sometimes ranking slightly higher, but clinical differences are modest. PubMedPMC
- Dual SGLT1/2 vs selective SGLT2 (head-to-head): In a randomized, double-blind trial (8 weeks) directly comparing sotagliflozin (SGLT1/2) vs empagliflozin (SGLT2-selective), overall glycemic control was comparable; the dual inhibitor reduced breakfast post-prandial glucose and insulin more (consistent with intestinal SGLT1 blockade), but this effect waned later in the day. PMC
- Mechanism for the post-prandial benefit: Human studies show SGLT1 inhibition delays intestinal glucose absorption, lowers post-meal glucose and insulin, reduces GIP, and raises GLP-1, explaining the breakfast spike blunting seen with sotagliflozin. PMCPubMed
- Any proof that SGLT1 adds more A1c reduction? Not convincingly. Reviews and meta-analyses of sotagliflozin emphasize cardiorenal outcomes; on glycemia, A1c benefits look similar to SGLT2-only agents, with the extra benefit mainly in post-prandial excursions, not a clearly larger A1c drop. PMC
- Safety nuance: Because SGLT1 is intestinal, dual inhibition more often causes GI effects (e.g., diarrhea) than SGLT2-selective drugs, although most events are mild. New England Journal of Medicine
Practical takeaway
If your goal is lower A1c, either approach works similarly on average. If a patient’s main problem is post-meal spikes(high PPG despite reasonable fasting readings), a dual SGLT1/2 agent may offer an added PPG benefit—but not necessarily a bigger A1c reduction—and with a bit more GI side-effect risk.