It’s the HR compared to GLP-1RAs! Compared to metformin or standard of care, the HR is about 0.5 in other papers. It’s massive.
Not sure if this has been posted already (let me know ) and not sure if to post this here or in the Cardiovascular Health thread…
Sotagliflozin, a drug recently approved by the Food and Drug Administration to treat type 2 diabetes and kidney disease with additional cardiovascular risk factors, can significantly reduce heart attack and stroke among these patients, according to results from an international clinical trial led by a Mount Sinai researcher.
Here’s the paper published yesterday: Effect of sotagliflozin on major adverse cardiovascular events: a prespecified secondary analysis of the SCORED randomised trial
Sodium–glucose co-transporter (SGLT)-2 inhibitors have shown consistent benefit in improving heart failure-related outcomes but not ischaemic cardiovascular events such as myocardial infarction or stroke. We assessed if the dual SGLT1/2 inhibitor sotagliflozin improves ischaemic outcomes.
We did a prespecified secondary analysis of the SCORED trial, which was a double-blind, placebo-controlled, randomised clinical trial enrolling patients (aged ≥18 years) with type 2 diabetes, chronic kidney disease (estimated glomerular filtration rate [eGFR] 25–60 mL/min per 1·73 m2), and additional cardiovascular risk factors.
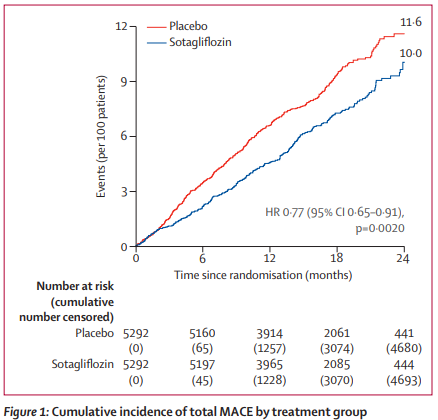
Patients in the sotagliflozin group had a significantly lower rate of total MACE than those in the placebo group (4·8 events per 100 person-years vs 6·3 events per 100 person-years; hazard ratio [HR] 0·77 [95% CI 0·65–0·91]; p=0·0020). Interaction analyses suggested a consistent effect of sotagliflozin on total MACE among stratified subgroups without evidence of heterogeneity. Additionally, sotagliflozin significantly reduced the rate of myocardial infarction (1·8 events per 100 person-years vs 2·7 events per 100 person-years; HR 0·68 [0·52–0·89]; p=0·0041) and stroke (1·2 events per 100 person-years vs 1·8 events per 100 person-years; HR 0·66 [0·48–0·91]; p=0·012) compared with placebo.
Sotagliflozin reduced MACE, with independent reductions in myocardial infarction and stroke, among patients with type 2 diabetes, chronic kidney disease, and additional cardiovascular risk. The ischaemic benefit on both myocardial infarction and stroke has not been previously observed with other SGLT inhibitors and warrants investigation of combined SGLT1 and SGLT2 inhibition as a possible underlying mechanism.
Comment in the Lancet: Sodium–glucose co-transporter inhibitors—who would have guessed?
“Prediction is very difficult, especially about the future”; whether it is the Nobel laureate Niels Bohr or others who have spoken on this matter, this is definitely true and a demanding reality in medical research.1 For the pharmaceutical industry, predicting the effectiveness of drugs is a major concern, and finding a blockbuster drug is challenging but highly warranted. Few people had predicted the broad effects of the sodium–glucose co-transporter (SGLT) inhibitor class when initially developing a diabetes drug that increased glucose excretion in urine. In the early phases of developement, SGLT inhibitors received little interest and attention even among diabetologists, as increased glucose in urine was considered to give unpleasant side-effects. Fortunately, several pharmaceutical companies developed and investigated their SGLT inhibitor compounds, including cardiovascular safety profiles. Today, the well known era of the SGLT inhibitor class, with extension of their diabetes indication to the prevention and treatment of heart failure and kidney disease even in patients without diabetes, is remarkable.
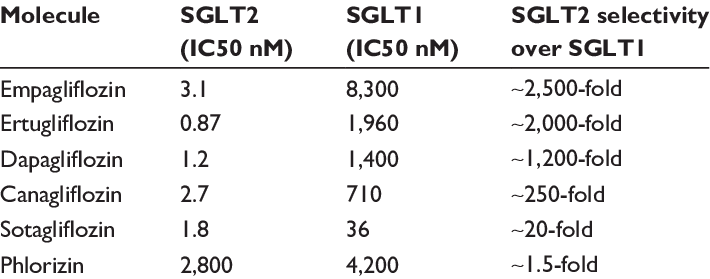
Canagliflozin is also an SGLT1 inhibitor but way less than sotagliflozin:
Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: evidence to date
Before everyone jumps to sotagliflozin: this paper did not find differences between sotagliflozin and other SGLT2 inhibitors other than an “increased risk of AKI in sotagliflozin” vs dapa and empa, which “may dilute the preventive effect of SGLT2 blockade, compared with more SGLT2‐selective agents”.
acute kidney injury (AKI)
New AgelessRx Study: Canagliflozin’s Expanded Role in Longevity
Pilot study that some of us participated, preliminary report out on Valentine’s Day ![]()
Note that it’s interesting that while the study was small they saw “Same Effects with Reduced Dosing”
Within the longevity community, there have been whispers of a potential longevity drug that may rival Metformin as the new go-to for extending healthy lifespan. That drug is Canagliflozin, a medication FDA approved to treat diabetes just like Metformin.
Though the longevity promise has been published for years in both model organisms and observational studies in diabetics, no clinical trials have explored the potential of Canagliflozin as a geroprotective agent. Does it really work to promote healthy longevity in normative aging individuals? Is it safe for healthy, non-diabetics to take? And if so, what doses are most beneficial?
To evaluate Canagliflozin’s viability for longevity, AgelessRx’s Applied Science team launched a pilot study that examined its short term safety and effectiveness in healthy subjects—a critical first step before exploring whether its beneficial actions go beyond disease therapy to support disease prevention.
Study Design: Canagliflozin’s Effects Examined
Run by AgelessRx, this trial explored a group of 24 healthy subjects who received Canagliflozin off-label under one of two dosing regimens: either 100mg a day for 7 days, or 150mg every 48 hours over 7 days.
Each subject had their blood sugar levels measured around the clock with a continuous glucose monitor (CGM) and provided regular self-evaluations. The researchers measured participants’ sugar-urine content to determine Canagliflozin’s efficacy for promoting the elimination of excess sugar from the body, and by proxy, exploring its potential for targeting the longevity-promoting molecular pathways activated by caloric restriction.
Main Findings
- Notable Urine Sugar Elimination
Some subjects eliminated as much as 2.1 grams of sugar in a single urine sample, even 24-48 hours after stopping therapy. For the average healthy individual, this could translate to eliminating up to half a can of soda from the blood each day! - Same Effects with Reduced Dosing
Each dosing group eliminated about the same amount of sugar, regardless of the dose strength or frequency of administration. This suggests that every other day dosing is just as effective as daily, paving the way for personalized dosing schedules hand-tailored to fit the patient’s unique physiology and health goals. - No Notable Negative Effects
No participants displayed any notable side effects, such as hypoglycemia or hypotension, and all participants seemed to tolerate the medication well. - Personalized Response to Canagliflozin
There were substantial differences between individuals in how much sugar was eliminated in response to Canagliflozin. This highlights the importance of taking a personalized approach, as two people may respond differently to the same dose of medication, and there are likely no one-size-fits-all solutions.
As the Applied Science team initiates more studies, keep an eye out for a potential new Canagliflozin offering from AgelessRx, and explore our other scientific efforts on our Research Science page. Our team is always recruiting for the next study, and you could help champion a new wave of healthcare for yourself and others like you.
https://agelessrx.com/new-agelessrx-study-canagliflozins-expanded-role-in-longevity/?
This is great news, especially for people like me who tend to prefer weekly schedules.
This seems to imply that sota doesn’t have additional benefits, but your previous post indicates that it does, especially with respect to preventing adverse cardiovascular events.
On another note, this paper seems to reveal why inhibiting SGLT1 is beneficial, and puts Canagliflozin back on top for longevity benefits. I had always assumed the longevity benefits of Cana were almost entirely due to blocking SGLT2, but now I’m not so sure.
Interesting drug… one of my suppliers has this one.
Verteporfin is a second-generation porphyrin photosensitizer, which can be used for photodynamic therapy in the treatment of macular degeneration.
The YAP-TEAD inhibitor Verteporfin disrupts migration/invasion dynamics across GBM types. Short-term drug treatment diminishes infiltrative tumor growth in aggressive PDX. This FDA-approved drug confers survival benefit in PDX models.
Anti-invasive efficacy and survival benefit of the YAP-TEAD inhibitor verteporfin in preclinical glioblastoma models - PMC
Is YAP taz at the root of cancer?
YAP and TAZ are highly related transcriptional regulators pervasively activated in human malignancies. Recent work indicates that, remarkably, YAP/TAZ are essential for cancer initiation or growth of most solid tumors. Their activation induces cancer stem cells attributes, proliferation, chemoresistance and metastasis.
YAP/TAZ at the roots of cancer - PMC
Verteporfin reverses progestin resistance through YAP/TAZ-PI3K-Akt pathway in endometrial carcinoma
In one trial in a very particular population.
In humans so far, besides the above trial, sotagliflozin has not been proved to be superior to “pure” SGLT2 inhibitors (dapagliflozin and empagliflozin). The same goes for canagliflozin.
Mendelian randomization studies showed that both SGLT1 and SGLT2 inhibition are associated with longer lifespan in males.
Thanks for the clarifications!
What’s the latest thinking about cycling on and off SGLT2i when taking them for longevity?
It is probably best to keep taking them indefinitely but cycling a few months on for additional kidney (and brain protection) and then a month off for avoiding sarcopenia and infections should also work imo.
I don’t think that generally there is any strong consensus yet.
Its complex, from my perspective.
Cycling, generally, seems to be a good thing but the exact period of cycling is open to debate. An additional, under-discussed factor is the impact on sleep of SGLT2i medications; when I take them I’m waking up at least twice a night to go to the bathroom whereas I don’t wake up at all typically; so there is a sleep interruption / disruption factor that also has to be calculated in. I’m still working on refining the approach of only hydrating during the morning before noon to see if there is a way to minimize this, but success will likely be an individual thing.
Like most of these medications; the best results are likely to be achieved via individual testing and protocol optimization.
Are there any longer acting SGLT2 inhibitors? Would that be something desirable? A longer acting inhibitor would be better from a compliance pov.
I am just now learning dapagliflozin can contribute to Sarcopenia. I’m confused because it was recommended I switch from metformin to this for that very reason. Is this at least better than metformin?
@RapAdmin Oddly it does not increase my bathroom trips ever, day nor night…hmmm?
AIUI, SGLT2-i contribute to sarcopenia simply because they tend to induce a calorie deficit. If you are weight stable you don’t need to worry. This is similar to the hysteria on GLP-1s and muscle mass loss.
Interesting… I mean the entire action of this medication is getting you to pee out more sugar, which typically means more bathroom trips. Perhaps you’ve already got a good protocol of hydrating mostly in the morning. Are you using US generic, or Indian sourced generic?
Same with me. Dapag was barely noticable…but also did not lower my fasting glucose which is why I take them in the first place. Empag lowers my fasting glucose, but also makes me get up in the night.
People do not urinate more on SGLT2i (past the first weeks and return to baseline). These drugs are not diuretics. See: SGLT2 Inhibition: Neither a Diuretic nor a Natriuretic commenting on Water Conservation Overrides Osmotic Diuresis During SGLT2 Inhibition in Patients With Heart Failure.
Kinetic Steering of Amyloid Formation and Polymorphism by Canagliflozin, a Type-2 Diabetes Drug 2025
Amyloid formation is involved in widespread health conditions such as Alzheimer’s disease, Parkinson’s disease, and type-2 diabetes. Amyloid fibrils have a similar cross-β architecture, but fibrils formed by a single protein sequence can have diverse structures, varying with time, self-assembly conditions, and sequence modifications. Fibril structure has been proposed to be diagnostic of disease, but why different structures result under different conditions, especially in vitro, remains elusive. We previously identified a small molecule, YX-I-1, which inhibits in vitro amyloid formation by islet amyloid polypeptide (IAPP), a peptide hormone whose amyloid formation is involved in type-2 diabetes. Here, using YX-I-1 as a lead, we identified regulator-approved drugs with similar structures by chemical similarity analysis and substructure searches, and monitored the effect of 24 of these potential ligands on IAPP amyloid assembly in vitro. We show that one such compound, canagliflozin (Invokana), a type-2 diabetes drug already in clinical use, can strongly delay the kinetics of IAPP amyloid formation, an activity independent of its intended mode of action (sodium-glucose linked transporter 2 (SGLT2) inhibitor) that may have important therapeutic implications. Combining analysis of amyloid self-assembly kinetics, biophysical characterization of monomer and fibril binding, and cryo-EM of the assembly products, we show that YX-I-1 and canagliflozin target IAPP early in aggregation, remodeling the energy landscape of primary nucleation and profoundly altering the resulting fibril structures. Early binding events thus imprint long-lasting effects on the amyloid structures that form.
However, two of the small molecules – doxazosin, an alpha-1 blocker used to treat hypertension and benign prostatic hyperplasia, and canagliflozin, one of the three most widely used flozins for treatment of type-2 diabetes via its action as an SGLT2 inhibitor – caused a pronounced and statistically significant inhibition (fold-change in half-time of 1.61 ± 0.29, p < 0.0001 and 1.55 ± 0.22, p < 0.0001, respectively), comparable to the inhibition observed for YX-I-1 (fold-change in halftime of 1.49 ± 0.18, p < 0.0001) (Table S7, Fig. S6). Canagliflozin and doxazosin are structurally distinct, but both possess a high degree of structural similarity to YX-I-1 enantiomer 1 as assessed by ROCS, with ComboScores of 0.759 (Table S1) and 0.864 (Table S2), respectively. The presence of shared tetrahydropyran and phenyl substructures makes this similarity particularly obvious for canagliflozin (Fig. 1c, Fig. 2c), but comparison of the ComboScores indicates that doxazosin nonetheless has a higher overall degree of shape and pharmacophoric similarity to YX-I-1, despite the aligned substructures being different (Fig. 2c).
Dapagliflozin (Fig. 5d), one of the closest analogs of canagliflozin in the screening set, showed a pattern of binding similar to canagliflozin, with greater attenuation of the aromatic protons compared to the aliphatic protons, but binding was weaker overall and inversion of the aromatic peaks did not occur.
Here, we have investigated whether regulator-approved small molecule drugs can be repurposed as inhibitors of IAPP amyloid formation, building upon our previous work that identified YX-I-1 as a lead26. Virtual screening, ThT assays and biophysical characterization successfully identified two widely used FDA-approved drugs, canagliflozin and doxazosin, as inhibitors of IAPP amyloid formation. Canagliflozin, currently used as a third-line type-2 diabetes medication with an intended mode of action (SGLT2 inhibitor) unrelated to islet amyloid, had the strongest inhibitory effect of all molecules tested. Whether treatment with canagliflozin provides additional clinically relevant benefits through an effect on IAPP fibrillization is unknown, although canagliflozin has been shown to improve pancreatic β-cell function via an uncharacterized SGLT2-independent mechanism. On the other hand, canagliflozin may currently be given too late in disease progression to take advantage of any therapeutic benefits by inhibiting IAPP amyloid formation. In addition, ex vivo structure determination of IAPP fibril deposits and clinical/animal studies will be required to determine whether canagliflozin treatment results in a change in IAPP fibril polymorphism in patients, and whether this affects disease outcomes. Doxazosin, on the other hand, is not currently used as a type-2 diabetes medication, but is widely used for conditions such as benign prostatic hyperplasia, and may also affect IAPP aggregation if administered to diabetic people with these conditions. As canagliflozin and doxazosin are both widely used drugs, and could be rapidly repurposed as amyloid-targeting type-2 diabetes treatments, the clinical effects of any current interactions with islet amyloid and the effects of early treatment in preor early-stage diabetic individuals should be investigated as a matter of urgency.
@DrFraser: I don’t understand the whole paper, but it’s one more datapoint in favor of SGLT2i (and specifically canagliflozin?) and alpha-blockers (and specifically doxazosin?). TBC…