What’s really interesting is that they used empagliflozin in this study, which (correct me if I’m wrong) is the most specific for SGLT2, so this suggests that the “off-target” target is NOT SGLT1.
Confirmation that SGLT2i might protect the liver as well, at least in diabetic people: Comparative effectiveness of sodium-glucose cotransporter-2 inhibitors and dipeptidyl peptidase-4 inhibitors in improvement of fatty liver index in patients with type 2 diabetes mellitus and metabolic dysfunction-associated steatotic liver disease: A retrospective nationwide claims database study in Japan 2024
This study aimed to evaluate the effectiveness of SGLT2i compared with that of dipeptidyl peptidase-4 inhibitors (DPP4i) in improving the fatty liver index (FLI) in patients with type 2 diabetes mellitus (T2DM) and metabolic dysfunction-associated steatotic liver disease (MASLD).
SGLT2i showed a higher incidence of improvement in the FLI (≥30%, ≥40% and ≥50% reduction from baseline FLI) compared with DPP4i, and the weighted hazard ratios were 1.27 (95% CI 1.18-1.38), 1.24 (95% CI 1.13-1.37) and 1.19 (95% CI 1.05-1.33), respectively. SGLT2i indicated a greater decreased in FLI values compared with DPP4i at up to 3 years of the follow-up period.
SGLT2is use appeared to be associated with a greater improvement of the FLI than DPP4i use in patients with T2DM and MASLD.
The ongoing trials will tell us if SGLT2i also protect the liver of people without diabetes. ![]()
Gout: one more indication for SGLT2i? See: Empagliflozin and Risk of Incident Gout: Analysis from the EMPagliflozin Comparative Effectiveness and SafEty (EMPRISE) Cohort Study 2024
Over a mean follow-up period of 8 months on treatment, the risk of gout was lower in patients initiating empagliflozin compared to DPP4i (HR = 0.69: 95% CI (0.60–0.79); RD = − 2.27: 95% CI (− 3.08, 1.46)) or GLP-1RA (HR = 0.83: 95% CI (0.73–0.94); RD = − 0.99: 95% CI (− 1.66, − 0.32)).
SGLT2 and SGLT1 inhibition play protective roles in CSVD development. The SGLT2 inhibition could lower the risk of SVS and improve the integrity of white matter microstructure via modulating the level of 4-acetamidobutanoate and cholesterol metabolism.
No risk (or even small benefit) of taking SGLT2i if you suffer from asthma: Association of novel antihyperglycemic drugs versus metformin with decrease in asthma exacerbations 2024
While DPP-4 Is and GLP-1 RAs were associated with poorer control of asthma compared with metformin, SGLT-2 Is offered asthma control comparable to that of metformin.
More evidence for combining GLP-1 RAs with SGLT2is? Combined sodium-glucose-transporters inhibitors and glucagon-like-peptide receptor agonist compared with monotherapy improves long-term survival: A Real-World Registry 2024
The combination therapy of sodium glucose cotransporter 2 inhibitor and glucagon like peptide 1 receptor agonist with respect to monotherapy reduces the risk of heart failure and all causes of mortality.
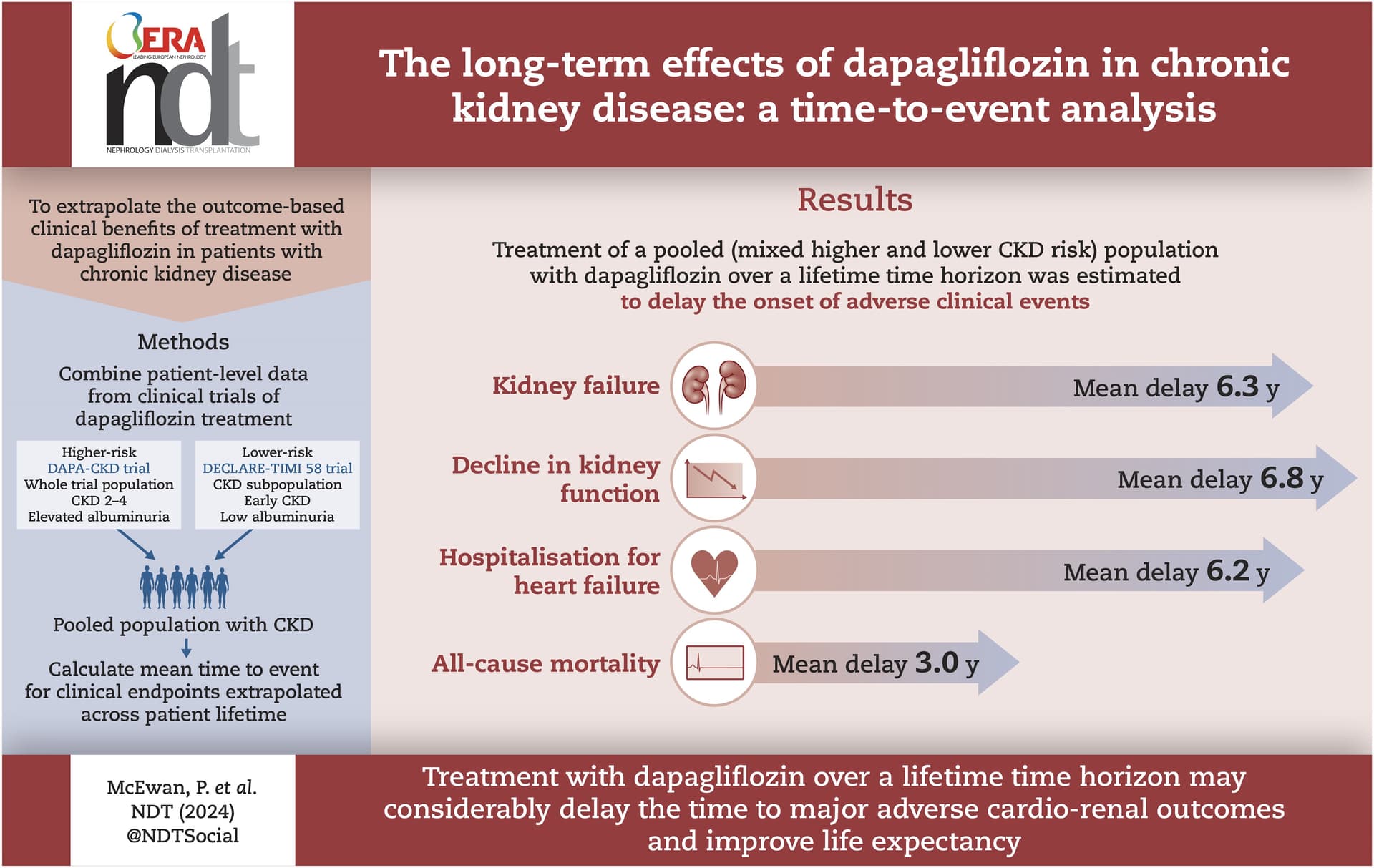
The long-term effects of dapagliflozin in chronic kidney disease: a time-to-event analysis 2024
In the DAPA-CKD and pooled CKD populations, treatment with dapagliflozin delayed time to first event for kidney failure, all-cause mortality, sustained decline in kidney function, and hospitalisation for heart failure.
Treatment with dapagliflozin over a lifetime time horizon may considerably delay the mean time to adverse clinical outcomes for patients who would go on to experience them, including those at modest risk of progression.
Has anyone tried as low of a dose of Jardiance as 5mg? I’m taking 10mg and debating cutting in half.
I’m taking 6.25mg as that’s the dose which doesn’t drop my blood sugar too low when I take it in the morning.
Edit:
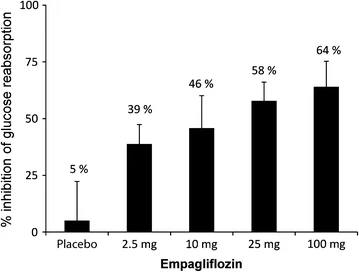
Based on this study Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Multiple Rising Doses of Empagliflozin in Patients with Type 2 Diabetes Mellitus | Diabetes Therapy (springer.com) it appears that the difference in glucose reabsorption inhibition should not be significantly different between 6.25mg and 10mg, ranging at ~40% inhibition compared to placebo.
Dr. Stanfied said he would consider adding 10mg Empagliflozin at the end here.
Just saw the video myself. Given the data of empagliflozin’s effect on kidney and cardiovascular health, it seems like a no-brainer to add at this point.
Agreed. I’ve been taking it for two years.
Have you ever experienced low blood sugar on Jardiance?
No. I just had some labs and my fasted was 86. A1C 5.1. Fasting insulin 3.5
I take the higher dose (25mg) of empagliflozin since it’s apparent that the potentially beneficial biologic effects aren’t provided strictly by glucose-lowering but by “off-target” effects; therefore, it may (or may not) be a faulty assumption that just because a half-dose works as well for glucose excretion, that it works just as well for everything else.
10 mg was pretty much equivalent in terms of outcomes in the heart failure trial though.
Yeah, I’m not sure if 25mg is better than 10mg, but I’m also 200 lbs (mostly lean mass), so I speculate that I may not get all the benefit of the 10mg dose that a smaller person might receive. I’m certainly open at any time to splitting the tabs in half to save money if I become solidly convinced that the 25mg dose is a waste.
At 12.5 mg, you’re getting 25% more than most people who start out at 10 mg. ![]()
As canagliflozin reduced lifespan in female mice:
- Did clinical trials show such a gender effect for T2D, CKD, HF or any other condition?
- Does the remark mean females should use a lower dose?
Checking this, it looks like indeed women might benefit less from SGLT2 than men:
- “The relative risk reduction in the primary composite cardiac outcome with SGLT2i over placebo therapy were significantly lower for women as compared to men in the clinical trials included in our meta-analysis. The event rate reductions for hospitalization for heart failure, cardiovascular death, and overall mortality were significantly lower in women across all the trials in the SGLT2i treatment group.” (Sglt2 Inhibitors In Women And Cardiovascular Outcomes - Meta-analysis Of Sex Differences In Eleven Randomized Clinical Trials 2024)
- “SGLT2 inhibitor was associated with decreased risks of cardiorenal outcomes similarly in both sexes.” (Sex differences for the efficacy of sodium-glucose cotransporter 2 inhibitors on cardiorenal outcomes: a systematic review and meta-analysis 2023)
- “SGLT-2is reduce the risk of primary composite outcomes in patients with HF, regardless of sex; however, the benefits were less pronounced in women.” (Sex differences in cardiovascular outcomes of SGLT-2 inhibitors in heart failure randomized controlled trials: A systematic review and meta-analysis 2023)
- “In contrast to previous findings, our updated meta-analysis suggests that women and men experience similar cardiorenal benefit in response to SGLT2 inhibition.” (Sodium-Glucose Cotransporter-2 Inhibition Benefits in Cardiorenal Risk in Men and Women 2022)
SGLT2 inhibitors reduced cardiovascular death by 14% in patients with heart failure (HR 0·86 [95% CI 0·79–0·93]), 15% in patients with type 2 diabetes (0·85 [0·79–0·91]), 11% in patients with chronic kidney disease (0·89 [0·82–0·96]), and 13% in patients with atherosclerotic cardiovascular disease (0·87 [0·78–0·97]).
SGLT2 inhibitors reduced heart failure events and cardiovascular death in patients with heart failure, type 2 diabetes, chronic kidney disease, and atherosclerotic cardiovascular disease. These effects were consistent across a wide range of subgroups within these populations. This supports the eligibility of a large population with cardiorenal-metabolic diseases for treatment with SGLT2 inhibitors.
Although the renal composite outcome did not differ between the two groups, the win ratio of the GLP-1RA-first group versus the SGLT2 inhibitor-first group was significant. These results suggest that, in GLP-1RA and SGLT2 inhibitor combination therapy, the addition of an SGLT2 inhibitor to baseline GLP-1RA treatment may lead to more favourable renal outcomes.
Protective Mechanisms of SGLTi in Ischemic Heart Disease 2024
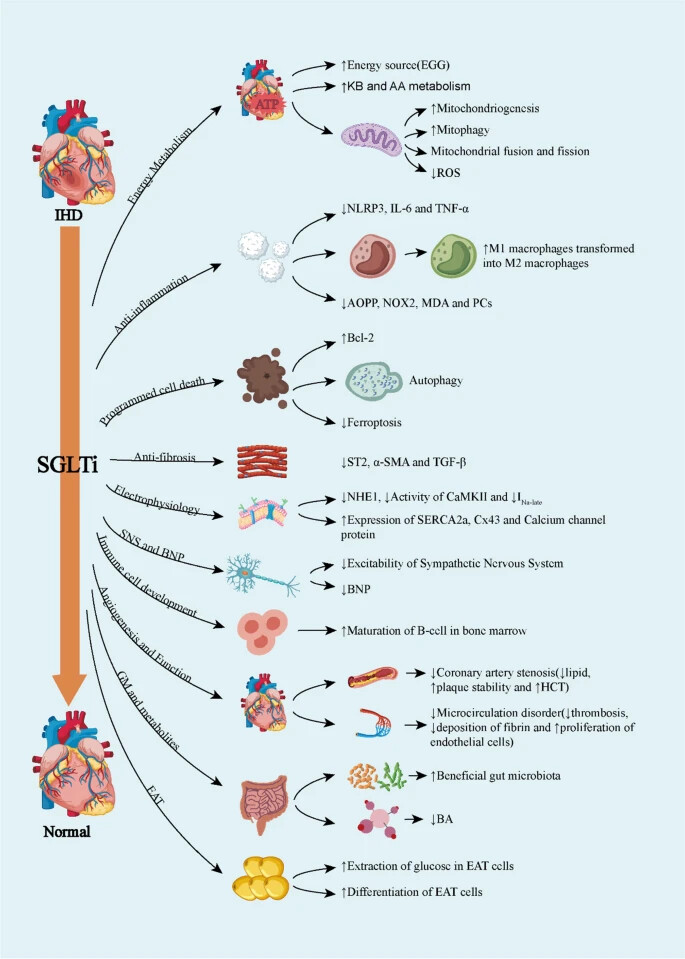
This review focuses on the mitochondrial mechanisms of empagliflozin, which underlie the anti-inflammatory and recover cellular functions in MetS cardiomyocytes, including stabilizing calcium concentration, mediating metabolic reprogramming, maintaining homeostasis of mitochondrial quantity and quality, stable mitochondrial DNA copy number, and repairing damaged mitochondrial DNA.
Safety of Empagliflozin: An Individual Participant-Level Data Meta-Analysis from Four Large Trials 2024
Total trial medication exposure was 19,727 patient-years for patients who received empagliflozin (n = 10,472) and 19,447 patient-years for placebo (n = 10,461). The percentages of patients with serious AEs, fatal AEs, and AEs leading to discontinuation were similar for both groups. The incidences of serious urinary tract infection and serious pyelonephritis or urosepsis were similar for both groups but higher for women taking empagliflozin versus placebo. Serious genital infections were not increased with empagliflozin versus placebo. There was a slight increase in ketoacidosis and serious volume depletion in patients who received empagliflozin versus placebo. The occurrence of serious acute kidney injury was lower with empagliflozin versus placebo. Empagliflozin was not associated with an increased incidence of severe hypoglycemia, bone fractures, or lower limb amputations. Empagliflozin is therefore considered safe in people without diabetes, the elderly, patients with very low estimated glomerular filtration rate, low body mass index, and HF. Safety is unaltered by blood pressure, concomitant medication for hypertension, HF, and immunosuppression.
Not sure if this has been posted before. This thread is quite long…
Patients ate more and yet (most) still lost weight after 90 weeks.
Eighty-six patients with type 2 diabetes (HbA1c 7.8 ± 0.8% [62 ± 9 mmol/mol], estimated glomerular filtration rate [eGFR] 89 ± 19 mL ⋅ min−1 ⋅ 1.73 m−2) received empagliflozin (25 mg/day) for 90 weeks with frequent (n = 11) assessments of body weight, eGFR, and fasting plasma glucose (FPG).
At week 90, weight loss averaged −3.2 ± 4.2 kg (corresponding to a median calorie deficit of 51 kcal/day [interquartile range (IQR) 112]). However, the observed calorie loss through glycosuria (206 kcal/day [IQR 90]) was predicted to result in a weight loss of –11.3 ± 3.1 kg, assuming no compensatory changes in energy intake. Thus, patients lost only 29 ± 41% of the weight loss predicted by their glycosuria; the model indicated that this difference was accounted for by a 13% (IQR 12) increase in calorie intake (269 kcal/day [IQR 258]) coupled with a 2% (IQR 5) increase in daily energy expenditure (due to diet-induced thermogenesis). This increased calorie intake was inversely related to baseline BMI (partial r = −0.34, P < 0.01) and positively to baseline eGFR (partial r = 0.29, P < 0.01).
Chronic glycosuria elicits an adaptive increase in energy intake. Combining SGLT2 inhibition with caloric restriction is expected to be associated with major weight loss.
We tested the hypothesis that EMPA would promote and increase longevity and physical activity ¶ by providing adult virgin female Drosophila with either DMSO (vehicle) or EMPA in the diet at concentrations of 5 μM, 25 μM, and 50 μM. EMPA treatment resulted in a significant extension of median lifespan at all concentrations: 4% (p=0.049) for 5 μM, 24% (p=0.035) for 25 μM, and 8% (p=0.025) for 50 μM, compared with DMSO.
A higher concentration is not better for female flies, but +24% at the mid-range concentration! If the paper is correct, it might confirm that SGLT1 inhibition is not necessary for longevity benefits. (poke @Neo)
Kidney protective mechanisms of SGLT2 inhibitors: evidence for a hemodynamic effect 2024
In conclusion, mechanistic physiology studies and clinical trials both support glomerular hypertension as a final common pathway for CKD progression, which is directly targeted by SGLT2 inhibitors. This effect may, in part, explain the benefits of SGLT2 inhibitors in kidney disease across diverse CKD causes, including patients with and without diabetes. Rather than inducing anxiety, observation of an eGFR dip should generally reassure clinicians and patients that SGLT2 inhibition is achieving the desired hemodynamic effect and protecting long-term kidney function.
Nine cohort studies involving 82,654 patients were included. SGLT2 inhibitor use was associated with a significantly lower risk of all-cause mortality (RR 0.46, 95% CI 0.31–0.68, P < 0.0001; I2 = 98%) and heart failure hospitalization (RR 0.49, 95% CI 0.30–0.81, P = 0.006; I2 = 21%) compared to non-use. The mortality benefit remained significant in patients receiving anthracycline chemotherapy (RR 0.50, 95% CI 0.28–0.89, P = 0.02; I2 = 71%).
SGLT2 inhibitor therapy is associated with lower risks of all-cause mortality and heart failure hospitalization in patients with concomitant diabetes and cancer. These findings suggest that SGLT2 inhibitors may offer cardiovascular benefits in this high-risk population. Randomized controlled trials are needed to validate these findings and evaluate the safety and efficacy of SGLT2 inhibitors in specific cancer types and treatment regimens.
Do you know more about the cancer interactions more broadly of SGLTi?