I’m not much of an expert but 3 grams of chromium sound like a lot? Are you sure humans can tolerate that. I thought minerals are supposed to be taken in low doses.
That’s 3 grams of Ceylon cinnamon + micrograms of chromium. ![]()
I learned the hard way that a urinalysis will show +1 or +2 glucose if you’re taking Empagliflozin. I was searching all over the internet about it and finally figured out what it was from
This shows greater effects when taken daily so I don’t see any reason to take it every other day.
I take 10mg Empa, 40mg Telm, and 5mg Nebivolol and have been for quite some time now.
UK biobank study (![]() preprint
preprint ![]() ) suggesting that SGLT2i (cana, dapa, empa) extend lifespan: First report from Epiterna on the search for drugs that can extend human lifespan
) suggesting that SGLT2i (cana, dapa, empa) extend lifespan: First report from Epiterna on the search for drugs that can extend human lifespan
Another paper confirming that empa and dapa are equivalent in T2D: Cardiovascular outcomes between dapagliflozin versus empagliflozin in patients with diabetes mellitus 2024
There was no significant difference between the dapagliflozin and empagliflozin groups in the risk of MACE (3.7% vs. 4.8%, hazard ratio [HR], 1.31; 95% confidence interval [CI], 0.73–2.35; p = 0.349)
Dapagliflozin and empagliflozin showed no significant difference of real‐world clinical cardiovascular outcomes in patients with DM over a 3‐year period.
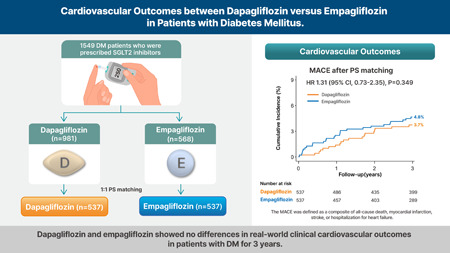
(if you look at a longer period of time, could dapa be better than empa?)
A potential pro-longevity pathway: SGLT2i improves kidney senescence by down-regulating the expression of LTBP2 in SAMP8 mice 2024
Together, these results reveal dapagliflozin can delay natural kidney senescence in non-diabetes environment; the mechanism may be through regulating the role of LTBP2.
Two reviews of the class:
Once these start coming off patent I wonder if we will see a meaningful uptake in longevity circles and broader health optimizer world…
… case is building in a nice way
Just wish we could see some rich MR data on longevity for SGLT2i
They’re already off-patent. But for whatever reason generics haven’t reached the market yet (despite approval in the US: Lupin Receives Tentative Approval for Empagliflozin Tablets - Lupin ).
I think that is - as the link says - just a tentative approval - and patent still has to expedite
See below for instance where there is tentative approval - but the FDA then lists patents going out to between Nov 2025 and others into 2034
Perhaps it’s only the Nov 2025 they have to wait for?
https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2019/212358Orig1s000_TAltr.pdf
Maybe. In the EU dapagliflozin is already approved as generic but I’ve never seen it: Dapagliflozin Viatris | European Medicines Agency
That second EU news piece seems similar, they just say:
”recommended granting approval”
Have we seen anything that actually is fully and completely approved
and not just tentatively or recommended
Or actually that is BOTH is approved by FDA, or EMA, etc AND has legal right to sell (not really regulated by medicine agency, but by the legal system/Justice Department).
They need both patent expirity and approval - it’s not enough for one
I work in a pharmacy, I’ve never seen dapagliflozin as a generic. Only Forxiga. By the way, dapagliflozin is way more prescribed than empagliflozin ![]()
Yes it’s fully approved: Dapagliflozin Viatris | European Medicines Agency
Per Wikipedia:
A generic version of dapagliflozin was approved by the US FDA in February 2022,[61] but cannot be sold until October 2025.[62][63] A generic version was approved in Canada in May 2023.[64]
In January 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for a generic version of Forxiga, which has been authorized in the EU since November 2012.[65] Dapagliflozin Viatris was approved for medical used in the European Union in March 2023.[4]
In France? Globally empagliflozin is number one, especially in the US and in the UK. Forxiga (Dapagliflozin) left Korea a few months ago because of the competition and the end of its patent there: Astrazeneca to withdraw diabetes drug Forxiga from Korea | BioWorld
Yes in France! Where I work it is.
Thanks, so then I think we are back to that it does not look the patents have expired until October in EU and November in US?
Well, they certainly have expired in India a long time ago. ![]()
![]()
The topic is when SGLTi could ”go big”
Once these start coming off patent I wonder if we will see a meaningful uptake in longevity circles and broader health optimizer world…
… case is building in a nice way
for that I think it’s needs to be like rapa and metformin - and accessible in the US directly at reasonable prices (and similar with Europe)
still for pioneers (and risk takers) like you and a portion of others on this forum in India may be great
Might have been already posted at some point but worth hammering this one down
Empagliflozin for Heart Failure With Preserved Left Ventricular Ejection Fraction With and Without Diabetes
In patients with heart failure and a preserved ejection fraction enrolled in the EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction), empagliflozin significantly reduced the risk of heart failure outcomes irrespective of diabetes status at baseline.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.122.059785
In patients with heart failure and a preserved ejection fraction enrolled in the EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction), empagliflozin significantly reduced the risk of heart failure outcomes irrespective of diabetes status at baseline.